| Citation: | Jing Li, Chang-ling Liu, Neng-you Wu, Xiao-qing Xu, Gao-wei Hu, Yan-long Li, Qing-guo Meng, 2022. Identification of functionally active aerobic methanotrophs and their methane oxidation potential in sediments from the Shenhu Area, South China Sea, China Geology, 5, 285-292. doi: 10.31035/cg2022011 |
Identification of functionally active aerobic methanotrophs and their methane oxidation potential in sediments from the Shenhu Area, South China Sea
-
Abstract
Large amounts of gas hydrate are distributed in the northern slope of the South China Sea, which is a potential threat of methane leakage. Aerobic methane oxidation by methanotrophs, significant methane biotransformation that occurs in sediment surface and water column, can effectively reduce atmospheric emission of hydrate-decomposed methane. To identify active aerobic methanotrophs and their methane oxidation potential in sediments from the Shenhu Area in the South China Sea, multi-day enrichment incubations were conducted in this study. The results show that the methane oxidation rates in the studied sediments were 2.03‒2.36 μmol/gdw/d, which were higher than those obtained by sediment incubations from other areas in marine ecosystems. Thus the authors suspect that the methane oxidation potential of methanotrophs was relatively higher in sediments from the Shenhu Area. After the incubations family Methylococcaea (type I methanotrophs) mainly consisted of genus Methylobacter and Methylococcaea_Other were predominant with an increased proportion of 70.3%, whereas Methylocaldum decreased simultaneously in the incubated sediments. Collectively, this study may help to gain a better understanding of the methane biotransformation in the Shenhu Area.
-

-
References
Beaudoin YC, Waite W, Boswell R, Dallimore SR. 2014. Frozen Heat: A UNEP Global Outlook on Methane Gas Hydrates. United Nations Environment Programme, GRID–Arendal, 29. doi: 10.1039/C1030EE00203H. Boetius A, Wenzhöfer F. 2013. Seafloor oxygen consumption fuelled by methane from cold seeps. Nature Geoscience, 6(9), 725–734. doi: 10.1038/NGEO1926. Bowman J. 2006. The methanotrophs—the families Methylococcaceae and Methylocystaceae. The Prokaryotes, Springer, 5, 266–289. doi: 10.1007/0-387-30745-1_15. Bowman JP. 2013. The Family Methylococcaceae. Springer Berlin Heidelberg, 411‒440. doi: 10.1007/978-3-642-38922-1_237. Bussmann I, Rahalkar M, Schink B. 2010. Cultivation of methanotrophic bacteria in opposing gradients of methane and oxygen. Fems Microbiology Ecology, 56(3), 331–344. doi: 10.1111/j.1574-6941.2006.00076.x. Carrier V, Svenning MM, Gründger F, Niemann H, Dessandier PA, Panieri G, Kalenitchenko D. 2020. The impact of methane on microbial communities at marine arctic gas hydrate bearing sediment. Frontiers in Microbiology, 11, 1932. doi: 10.3389/fmicb.2020.01932. Case DH, Ijiri A, Morono Y, Tavormina P, Orphan VJ, Inagaki F. 2017. Aerobic and anaerobic methanotrophic communities associated with methane hydrates exposed on the seafloor: A high-pressure sampling and stable isotope-incubation experiment. Frontiers in Microbiology, 8, 2569. doi: 10.3389/fmicb.2017.02569. Chidambarampadmavathy K, Karthikeyan OP, Huerlimann R, Maes GE, Heimann K. 2016. Response of mixed methanotrophic consortia to different methane to oxygen ratios. Waste Management, 61, 220–228. doi: 10.1016/j.wasman.2016.11.007. Crossman ZM, Ineson P, Evershed RP. 2005. The use of 13C labelling of bacterial lipids in the characterisation of ambient methane-oxidising bacteria in soils. Organic Geochemistry, 36(5), 769–778. doi: 10.1016/j.orggeochem.2004.12.005. Dedysh SN, Belova SE, Bodelier P, Smirnova KV, Khmelenina VN, Chidthaisong A, Trotsenko YA, Liesack W, Dunfield PF. 2007. Methylocystis heyeri sp. nov., a novel type II methanotrophic bacterium possessing ‘signature’ fatty acids of type I methanotrophs. International Journal of Systematic and Evolutionary Microbiology, 57(3), 472‒479. doi:10.1099/ijs.0.64623-0. Duc NT, Crill P, Bastviken D. 2010. Implications of temperature and sediment characteristics on methane formation and oxidation in lake sediments. Biogeochemistry, 100(1), 185–196. doi: 10.1007/s10533-010-9415-8. Einola JKM, Kettunen RH, Rintala JA. 2007. Responses of methane oxidation to temperature and water content in cover soil of a boreal landfill. Soil Biology and Biochemistry, 39(5), 1156–1164. doi: 10.1016/j.soilbio.2006.12.022. Feng D, Qiu JW, Hu Y, Peckmann J, Guan H, Tong H, Chen C, Chen J, Gong S, Li N, Chen D. 2018. Cold seep systems in the South China Sea: An overview. Journal of Asian Earth Sciences, 168, 3–16. doi: 10.1016/j.jseaes.2018.09.021. Gebert J, Groengroeft A, Miehlich G. 2003. Kinetics of microbial landfill methane oxidation in biofilters. Waste Management, 23(7), 609–619. doi: 10.1016/S0956-053X(03)00105-3. Guan HX, Xu LF, Liu L, Li SZ, Feng JX, Tao J, Kuang ZG, Liang JQ, Wu NY. 2021. Lipid biomarker patterns reflect seepage activity and variable geochemical processes in sediments from the Haima cold seeps, South China Sea. Palaeogeography, Palaeoclimatology, Palaeoecology, 110742. doi: 10.1016/j.palaeo.2021.110742. Hu Y, Feng D, Peckmann J, Gong S, Liang Q, Wang H, Chen D. 2020. The impact of diffusive transport of methane on pore-water and sediment geochemistry constrained by authigenic enrichments of carbon, sulfur, and trace elements: A case study from the Shenhu area of the South China Sea. Chemical Geology, 553, 119805. doi: 10.1016/j.chemgeo.2020.119805. Knief C, Dunfield PF. 2005. Response and adaptation of different methanotrophic bacteria to low methane mixing ratios. Environmental Microbiology, 7(9), 1307–1317. doi: 10.1111/j.1462-2920.2005.00814.x. Krüger M, Treude T, Wolters H, Nauhaus K, Boetius A. 2005. Microbial methane turnover in different marine habitats. Palaeogeography, Palaeoclimatology, Palaeoecology, 227(1‒3), 6‒17. doi: 10.1016/j.palaeo.2005.04.031. Liang QY, Zhao J, Xia Z, Yang SX, Kang JH, Lin JQ, Lei ZS, Deng YN, Teng DQ. 2017. Distribution characteristics and influential factors of dissolved methane in sea water above gas hydrate area on the northern slope of the South China Sea. Earth Science Frontiers, 24(4), 89–101. doi: 10.13745/j.esf.yx.2016-12-40. Li J, Xu X, Liu C, Wu N, Sun Z, He X, Chen Y. 2021a. Active methanotrophs and their response to temperature in marine environments: An experimental study. Journal of Marine Science and Engineering, 9(11), 1261. doi: 10.3390/jmse9111261. Li J, Liu CL, Wu NY, He XL, Meng QG, Xu XX, Chen Y. 2021b. A review on microbial aerobic methane oxidaiton in marine environment. Marine Geology and Quaternary Geology, 41(5), 67–76 (in Chinese with English abstract). doi: 10.16562/j.cnki.0256-1492.2020112302. Mohanty SR, Bodelier PL, Conrad R. 2007. Effect of temperature on composition of the methanotrophic community in rice field and forest soil. Fems Microbiology Ecology, 62, 24–31. doi: 10.1111/j.1574-6941.2007.00370.x. Nguyen NL, Yu WJ, Yang HY, Kim JG, Jung MY, Park SJ, Roh SW, Rhee SK. 2017. A novel methanotroph in the genus Methylomonas that contains a distinct clade of soluble methane monooxygenase. Journal of Microbiology, 55(10), 775–782. doi: 10.1007/s12275-017-7317-3. Reddy KR, Rai RK, Green SJ, Chetri JK. 2019. Effect of temperature on methane oxidation and community composition in landfill cover soil. Journal of Industrial Microbiology and Biotechnology, 46(9‒10), 1283‒1295. doi: 10.1007/s10295-019-02217-y. Steinle L, Graves CA, Treude T, Ferré B, Biastoch A, Bussmann I, Berndt C, Krastel S, James RH, Behrens E. 2015. Water column methanotrophy controlled by a rapid oceanographic switch. Nature Geoscience, 8(5), 378–382. doi: 10.1038/NGEO2420. Taubert M, Grob C, Crombie A, Howat AM, Burns OJ, Weber M, Lott C, Kaster AK, Vollmers J, Jehmlich N. 2019. Communal metabolism by Methylococcaceae and Methylophilaceae is driving rapid aerobic methane oxidation in sediments of a shallow seep near Elba, Italy. Environmental microbiology, 21(10), 3780–3795. doi: 10.1111/1462-2920.14728. Valentine DL. 2010. Emerging topics in marine methane biogeochemistry. Annual Review of Marine Science, 3(1), 147–171. doi: 10.1146/annurev-marine-120709-142734. Wang ZP, Ineson P. 2003. Methane oxidation in a temperate coniferous forest soil: Effects of inorganic N. Soil Biology & Biochemistry, 35(3), 427–433. doi: 10.1016/S0038-0717(02)00294-8. Wei JG, Liang JQ, Lu JA, Zhang W, He YL. 2019. Characteristics and dynamics of gas hydrate systems in the northwestern South China Sea - Results of the fifth gas hydrate drilling expedition. Marine and Petroleum Geology, 110, 287–298. doi: 10.1016/j.marpetgeo.2019.07.028. Wu NY, Zhang HQ, Yang SX, Zhang GX, Liang JQ, Lu JA, Su X, Schultheiss P, Holland M, Zhu YH. 2011. Gas hydrate system of Shenhu area, northern South China Sea: Geochemical results. Journal of Geological Research, 370298. doi: 10.1155/2011/370298. Yang HL, Yu S, Lu HL. 2021. Iron-coupled anaerobic oxidation of methane in marine sediments: A review. Journal of Marine Science and Engineering, 9(8), 875. doi: 10.3390/jmse9080875. Zeng LL, Tian JQ, Chen H, Wu N, Yan ZY, Du LF, Shen Y, Wang X. 2019. Changes in methane oxidation ability and methanotrophic community composition across different climatic zones. Journal of Soils and Sediments, 19(2), 533–543. doi: 10.1007/s11368-018-2069-1. -
Access History

-
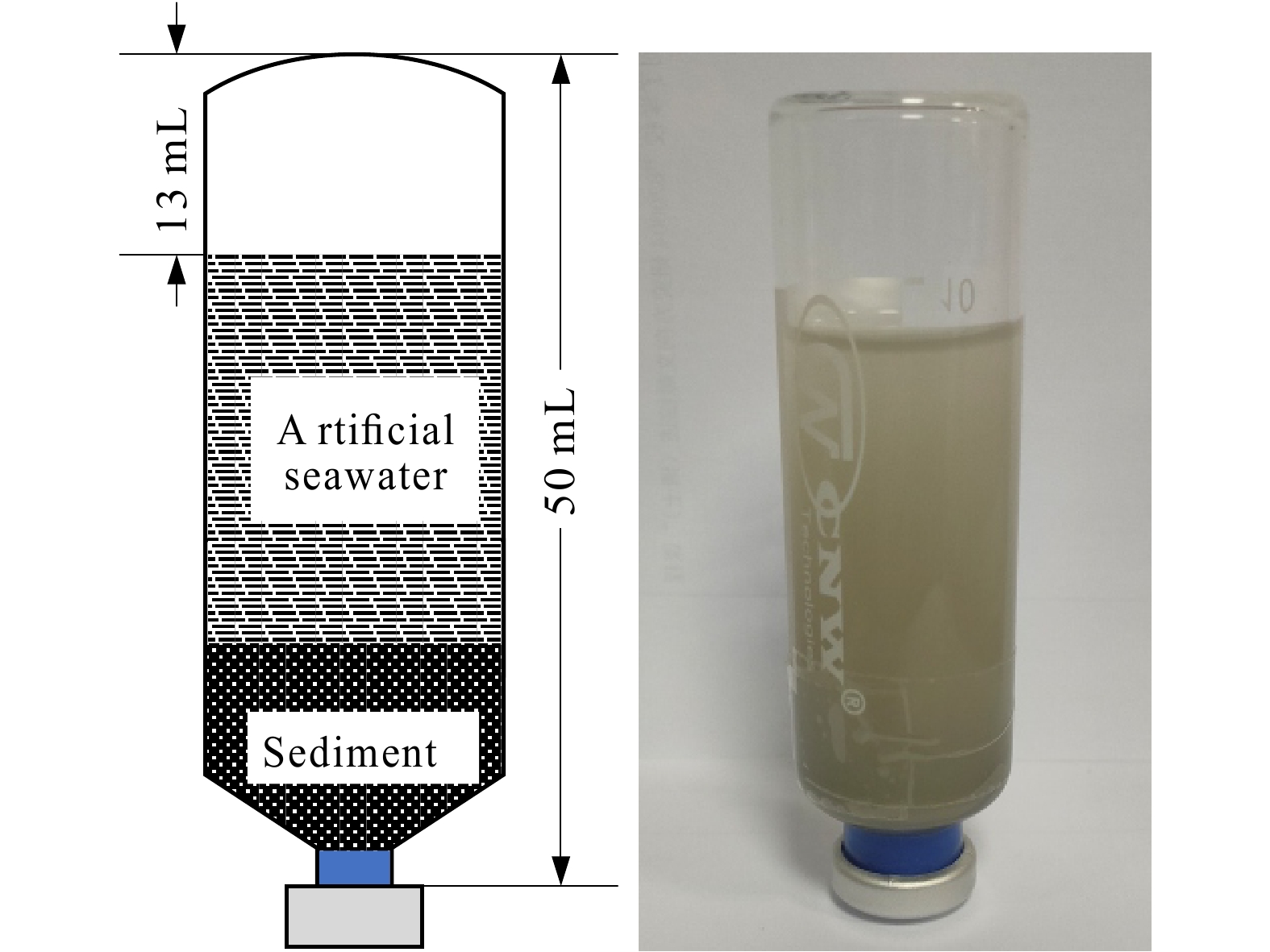
Figure 1.
Experimental culture system in this study.
-
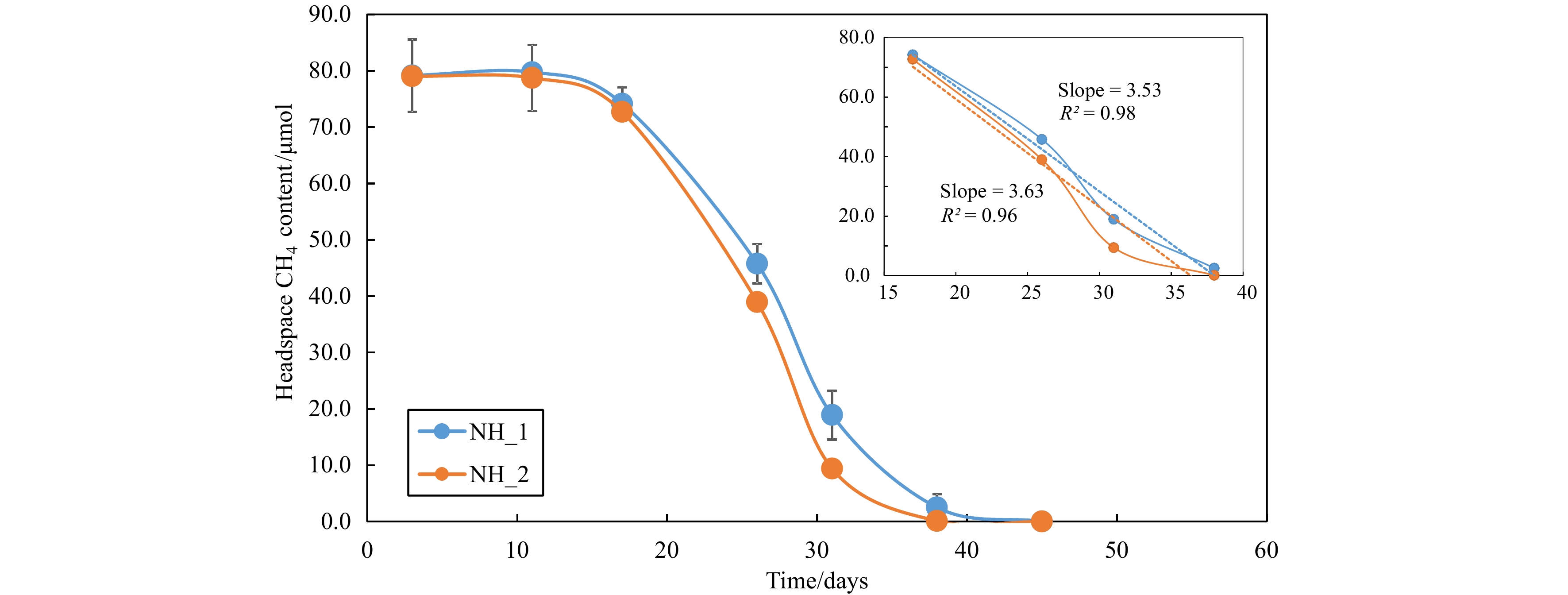
Figure 2.
Methane consumption by methanotrophs over the incubation time with NH_1 and NH_2. Linear regression of the steepest curves showed in at image top right.
-
Figure 3.
Distribution of FAMEs in studied sediment samples (m/z=74, internal standard). a‒NH_1_Original; b‒NH_1_Incubated_45days; c‒NH_2_Original; d‒NH_2_Incubated_45days.
-
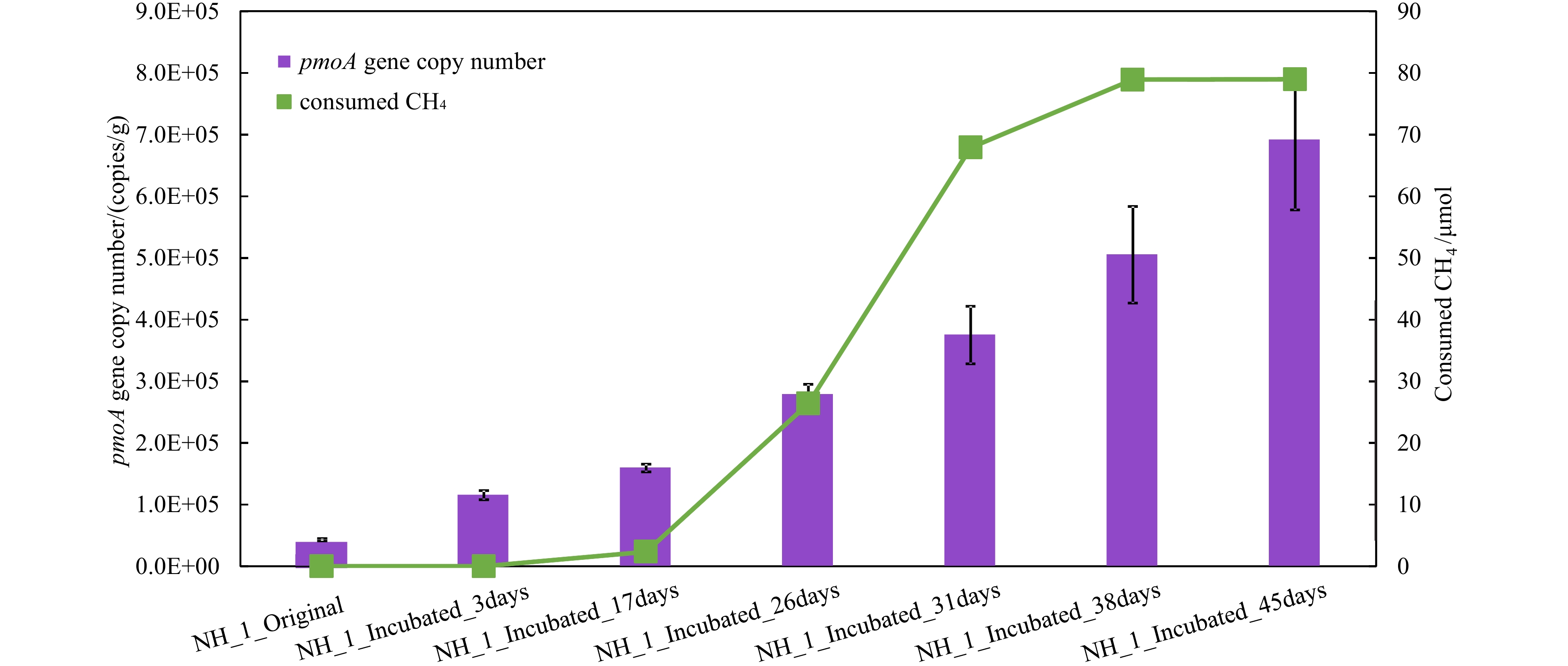
Figure 4.
pmoA gene copy number and CH4 content by methanotrophs over the incubation time of NH_1.
-
Figure 5.
Taxonomic composition of methanotrophic communities in studied sediment samples.




 DownLoad:
DownLoad: