| Citation: | Huan Huang, Chang-fu Chen, Xiao-jie Mo, Ding-ding Wu, Yan-ming Liu, Ming-zhu Liu, Hong-han Chen, 2021. Mechanisms of salt rejection at the ice-liquid interface during the freezing of pore fluids in the seasonal frozen soil area, China Geology, 4, 446-454. doi: 10.31035/cg2021059 |
Mechanisms of salt rejection at the ice-liquid interface during the freezing of pore fluids in the seasonal frozen soil area
-
Abstract
Seasonal frozen soil accounts for about 53.50% of the land area in China. Frozen soil is a complex multiphase system where ice, water, soil, and air coexist. The distribution and migration of salts in frozen soil during soil freezing are notably different from those in unfrozen soil areas. However, little knowledge is available about the process and mechanisms of salt migration in frozen soil. This study explores the mechanisms of salt migration at the ice-liquid interface during the freezing of pore fluids through batch experiments. The results are as follows. The solute concentrations of liquid and solid phases at the ice-liquid interface (
< span class="inline-formula-span" > < span class="inline-formula-span" > < span class="inline-formula-span" > $ {C}_{L}^{*} $ < /span > < img text_id='' class='formula-img' style='display:none;' src='cg2021059_M5.png'/ > < /span > < img text_id='' class='formula-img' style='display:none;' src='cg2021059_M3.png'/ > < /span > < img text_id='' class='formula-img' style='display:none;' src='cg2021059_M1.png'/ > < span class="inline-formula-span" > < span class="inline-formula-span" > < span class="inline-formula-span" > $ {C}_{S}^{*} $ < /span > < img text_id='' class='formula-img' style='display:none;' src='cg2021059_M6.png'/ > < /span > < img text_id='' class='formula-img' style='display:none;' src='cg2021059_M4.png'/ > < /span > < img text_id='' class='formula-img' style='display:none;' src='cg2021059_M2.png'/ > < span class="inline-formula-span" > < span class="inline-formula-span" > < span class="inline-formula-span" > $ {C}_{L}^{*} $ < /span > < img text_id='' class='formula-img' style='display:none;' src='cg2021059_M5.png'/ > < /span > < img text_id='' class='formula-img' style='display:none;' src='cg2021059_M3.png'/ > < /span > < img text_id='' class='formula-img' style='display:none;' src='cg2021059_M1.png'/ > < span class="inline-formula-span" > < span class="inline-formula-span" > < span class="inline-formula-span" > $ {C}_{S}^{*} $ < /span > < img text_id='' class='formula-img' style='display:none;' src='cg2021059_M6.png'/ > < /span > < img text_id='' class='formula-img' style='display:none;' src='cg2021059_M4.png'/ > < /span > < img text_id='' class='formula-img' style='display:none;' src='cg2021059_M2.png'/ > < span class="inline-formula-span" > < span class="inline-formula-span" > < span class="inline-formula-span" > $ {C}_{L}^{*} $ < /span > < img text_id='' class='formula-img' style='display:none;' src='cg2021059_M5.png'/ > < /span > < img text_id='' class='formula-img' style='display:none;' src='cg2021059_M3.png'/ > < /span > < img text_id='' class='formula-img' style='display:none;' src='cg2021059_M1.png'/ > < span class="inline-formula-span" > < span class="inline-formula-span" > < span class="inline-formula-span" > $ {C}_{S}^{*} $ < /span > < img text_id='' class='formula-img' style='display:none;' src='cg2021059_M6.png'/ > < /span > < img text_id='' class='formula-img' style='display:none;' src='cg2021059_M4.png'/ > < /span > < img text_id='' class='formula-img' style='display:none;' src='cg2021059_M2.png'/ > -

-
References
Akyurt M, Zaki G, Habeebullah B. 2002. Freezing phenomena in ice-water systems. Energy Conversion and Management, 43(14), 1773–1789. doi: 10.1016/S0196-8904(01)00129-7. Baker GC, Osterkamp TE. 1989. Salt redistribution during freezing of saline sand columns at constant rates. Water Resources Research, 25(8), 1825–1831. doi: 10.1029/WR025i008p01825. Bittelli M, Flury M, Campbell GS. 2003. A thermodielectric analyzer to measure the freezing and moisture characteristic of porous media. Water Resources Research, 39(2), SBH11–1. doi: 10.1029/2001WR000930. Deng Y, Jia ZD, Wei XX, Su HF, Guan ZC, Zhou J. 2012. Salt migration phenomena during phase transition in cooling water and its impact on leakage current. Proceedings of the Chinese Society for Electrical Engineering, 32(28), 184–191 (in Chinese with English abstract). Huang H, Liu MZ, Chen CF, Huang GX, Chen HH. 2019. Laboratory studies on nitrate redistribution during the freezing process of a water-saturated sand system. Environmental Science and Pollution Research, 26(14), 13818–13824. doi: 10.1007/s11356-018-3251-0. Jie WQ. 2010. Principle and Technology of Crystal Growth. China. Beijing: Science Press. 199, 457 (in Chinese). Konrad JM, Mccammon AW. 1990. Solute partitioning in freezing soils. Canadian Geotechnical Journal, 27(6), 726–736. doi: 10.1016/0148-9062(91)90104-T. Liu L, Xue Y, Zhang J. 1999. Brief introduction of application and research in freeze concentration technology. Chemical Indusrty and Engineering, (3), 151–156 (in Chinese with English abstract). Liu QS, Qiu TS. 2004. Study progress on remediation of contaminated soil and groundwater. Energy Environmental Protection, 18(005), 25–28 (in Chinese with English abstract). doi: 10.3969/j.issn.1006-8759.2004.05.007. Lorain O, Thiebaud P, Badorc E, Aurelle Y. 2001. Potential of freezing in wastewater treatment: Soluble pollutant applications. Water Research, 35(2), 541–547. doi: 10.1016/S0043-1354(00)00287-6. Lü HZ, Li CY, Shi YH, Zhao SN, Yang F, Wu Y, Song S. 2015. Pollutant distribution under different conditions in lake Uliangsuhai ice-water system. Journal of Lake Science, 27(6), 1151–1158 (in Chinese with English abstract). doi: 10.18307/2015.0621. Ma YP, Xu YH. 2008. Theory and Technology of Metal Solidification. China. Beijing, Metallurgical Industry Press. 61‒66 (in Chinese). Mtombeni T, Maree JP, Zvinowanda CM, Asante JKO, Oosthuizen FS, Louw WJ. 2013. Evaluation of the performance of a new freeze desalination technology. International Journal of Environmental Science and Technology, 10(3), 545–550. doi: 10.1007/s13762-013-0182-7. Ostroumov VE, Hoover R, Ostroumova NV, Vliet-Lanoë BV, Sorokovikov V. 2001. Redistribution of soluble components during ice segregation in freezing ground. Cold Regions Science & Technology, 32(2), 175–182. doi: 10.1016/S0165-232X(01)00031-3. O’Neill K, Miller RD. 1985. Exploration of a rigid ice model of frost heave. Water Resources Research, 21(3), 281–296. doi: 10.1029/WR021i003p00281. Panday S, Corapcioglu MY. 1991. Solute rejection in freezing soils. Water Resources Research, 27(1), 99–108. doi: 10.1029/90WR01785. Suh HS, Yun TS. 2018. Modification of capillary pressure by considering pore throat geometry with the effects of particle shape and packing features on water retention curves for uniformly graded sands. Computers and Geotechnics, 95, 129–136. doi: 10.1016/j.compgeo.2017.10.007. Terwilliger JP, Dizon SF. 1970. Salt rejection phenomena in the freezing of saline solutions. Chemical Engineering Science, 25(8), 1331–1349. doi: 10.1016/0009-2509(70)80010-0. Williams PM, Ahmad M, Connolly BS. 2013. Freeze desalination: An assessment of an ice maker machine for desalting brines. Desalination, 308(6), 219–224. doi: 10.1016/j.desal.2012.07.037. Xu XZ, Wang JC, Zhang LX. 2010. Frozen Soil Physics. China. Beijing, Science Press, 390( in Chinese). Xue S, Chen J, Tie M, Hui XJ, Zhang LN, Zhang Y. 2014. Ratio of haloacetic acids precursor in water-ice system during the freezing processes of water. China Environmental Science, 33(11), 2773–2780 (in Chinese with English abstract). doi: 10.3969/j.issn.1000-6923.2014.11.009. Yang P, Diao PC, Zhang T, Yang GP. 2021, A study of the influences of freezing temperature and thawing conditions on physical properties of marine soft soil before and after freezing-thawing. Hydrogeology and Engineering Geology, 48(1), 96–104. doi: 10.16030/j.cnki.issn.1000-3665.202003010 Yang H, Yao YX, Li HS. 2016. Experimental study on the effect of freezing seawater concentration factors. Technology of water treatment, (9), 68–72. doi: 10.16796/j.cnki.1000-3770.2016.09.014. Yu T, Ma J. 2005. Study on influence factors of ice crystal purity during freeze concentration process. Journal of Harbin University of Commerce: Natural Science Edition, 21(5), 572–578. doi: 10.3969/j.issn.1672-0946.2005.05.009. Zuo C. 2018. Effects of Different Freezing Conditions on the Redistribution of Benzene in Ice Water Two Phase. China. Beijing, China University of Geosciences (Beijing), Ph. D thesis. 1‒6, 42–44 (in Chinese with English abstract). Zhang LN. 2013. Changes of spectral characteristics of dissolved organic matter during water freezing. China. Shenyang, Liaoning University, Ph. D thesis. 46‒47 (in Chinese with English abstract). -
Access History

-
Figure 1.
Solute redistribution in the case that only diffusion occurs in the liquid phase during metal solidification. a–start of solidification; b‒initial transition stage; c–stable growth stage; d‒complete solidification; S–solid phase; L‒liquid phase (after Ma YP et al., 2008).
-
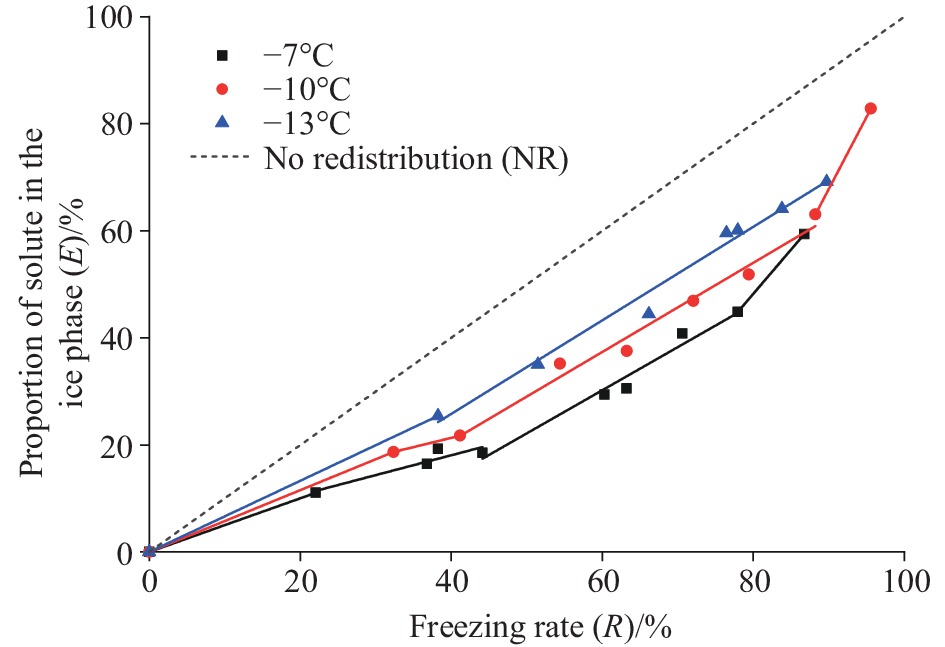
Figure 2.
E-R relationship of pore fluids at different freezing temperatures.
-
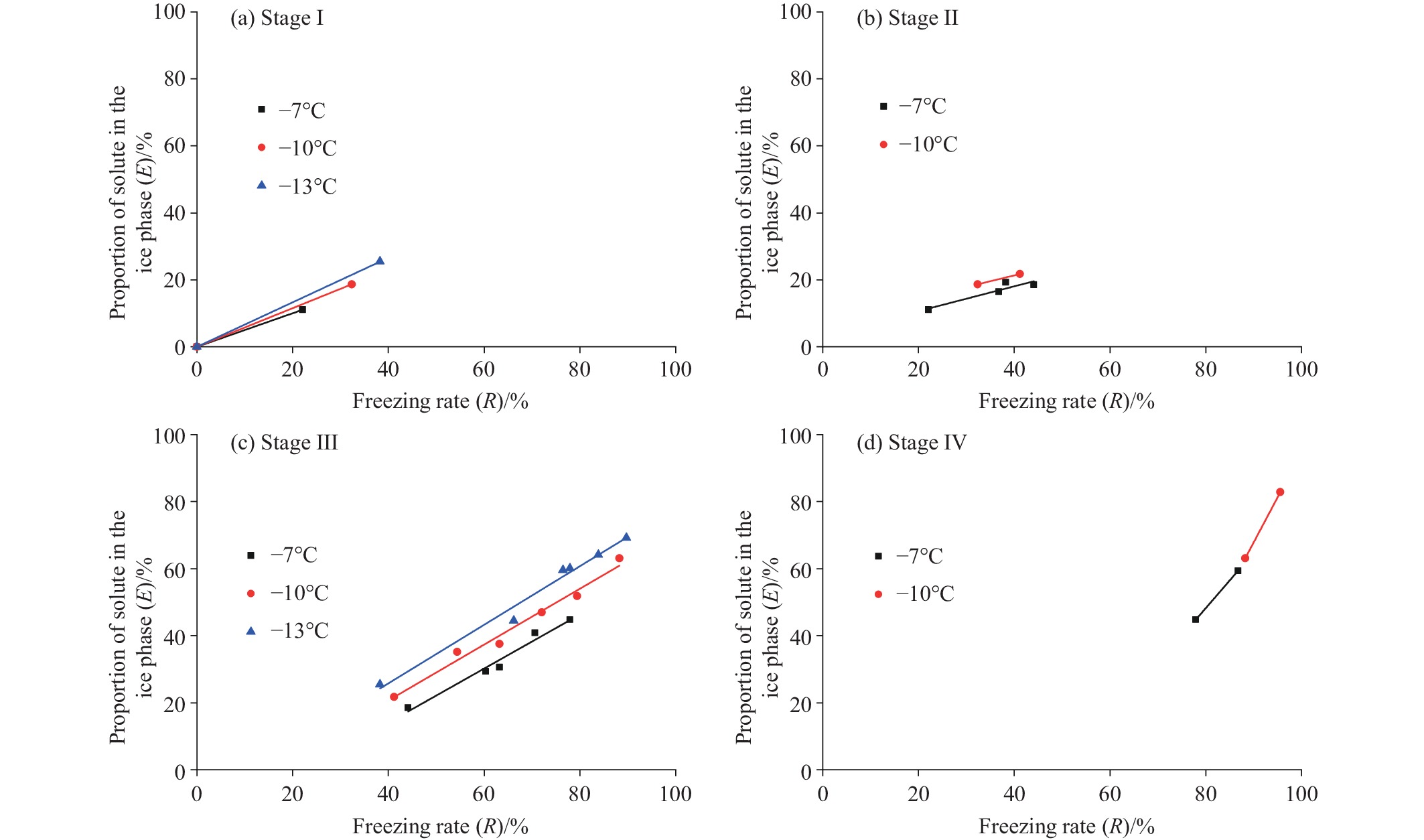
Figure 3.
E-R relationship (by stage) of the pore fluids at different freezing temperatures.
-
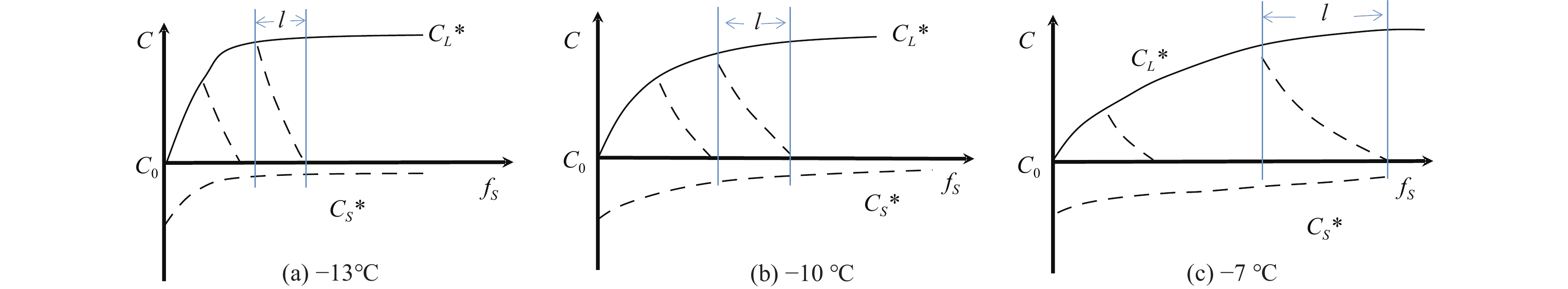
Figure 4.
Diagrams of solute enrichment characteristics at the freezing front of pore fluids under different freezing temperatures.
-
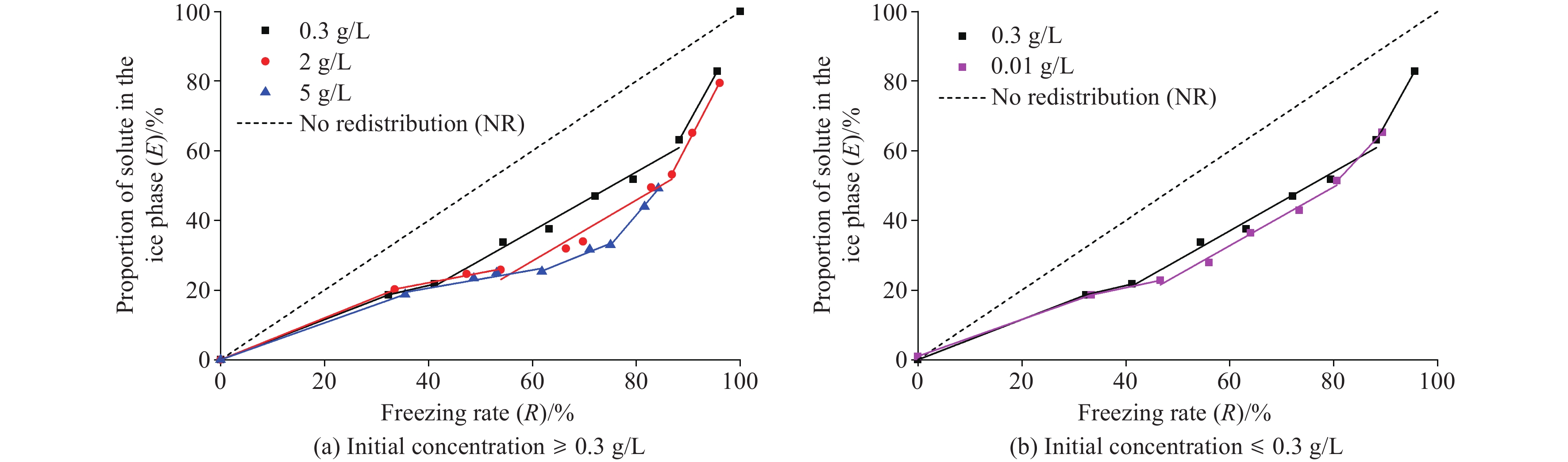
Figure 5.
E-R relationship during the freezing of pore fluids with different initial concentrations.
-
Figure 6.
E-R relationship (by stage) during the freezing of pore fluids with different initial concentrations.
-
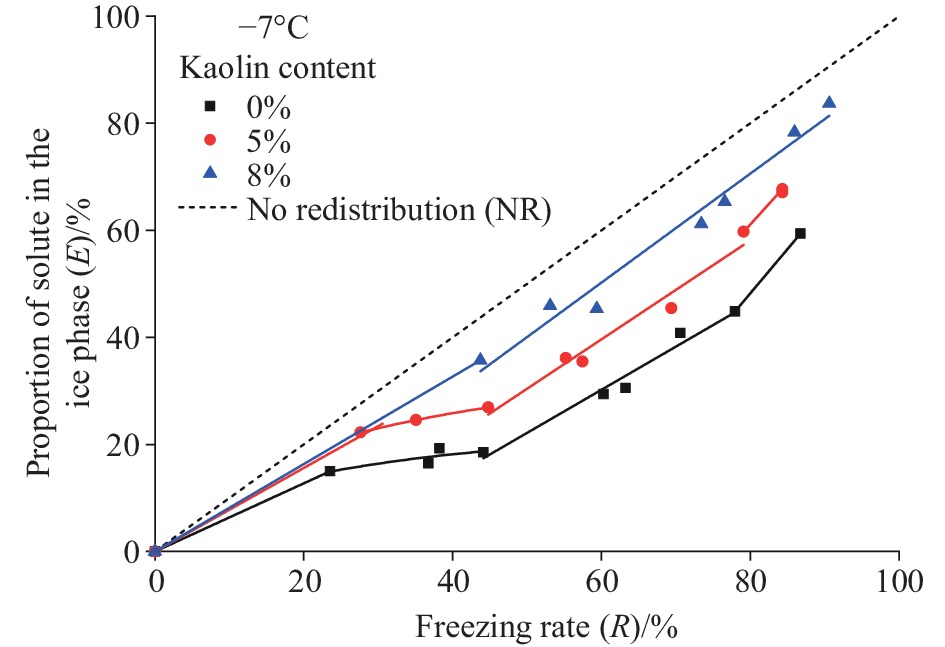
Figure 7.
E-R relationship of pore fluids under different particle-size distribution of media.
-
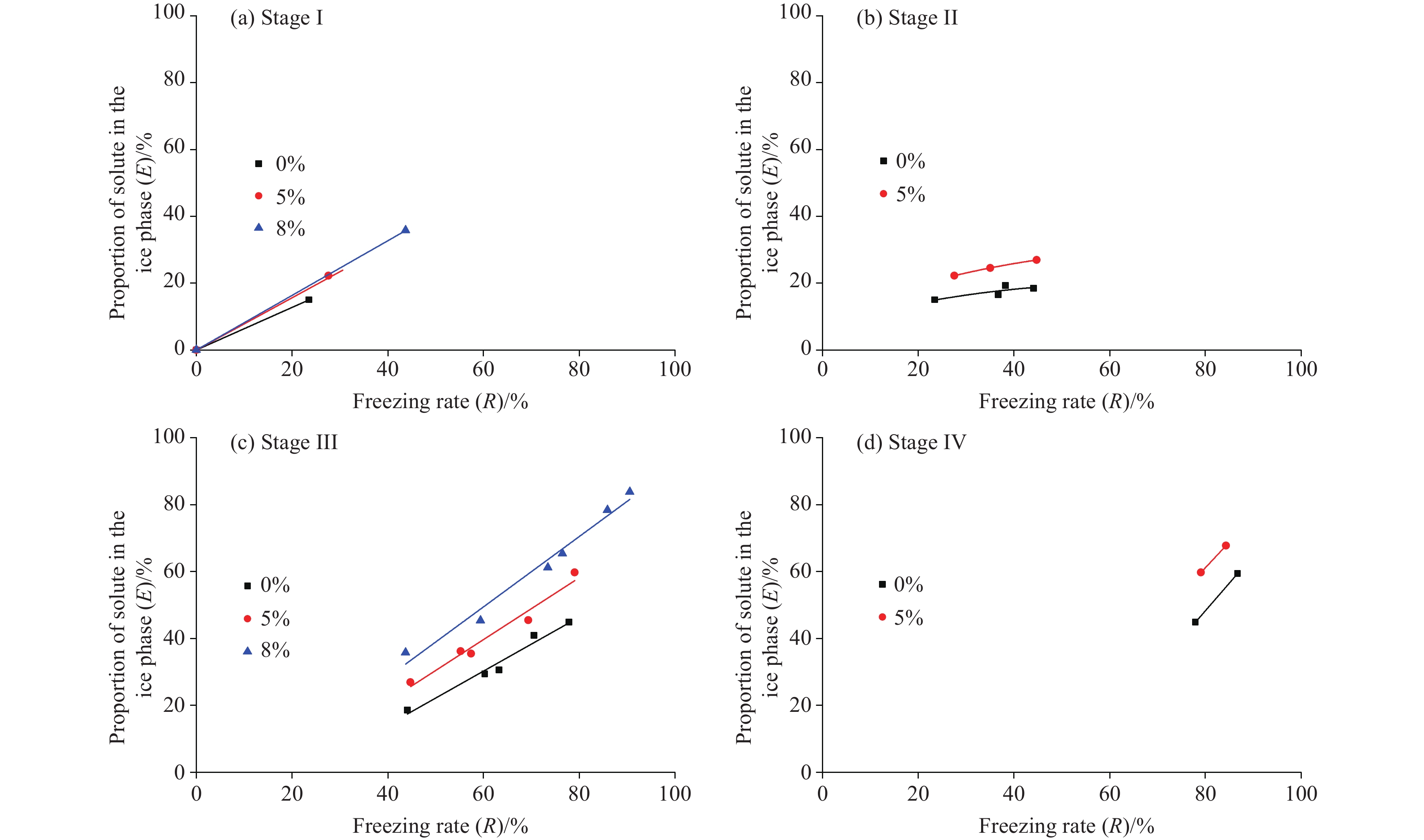
Figure 8.
E-R relationship (by stage) of pore fluids under different particle-size distribution of media.







 DownLoad:
DownLoad: