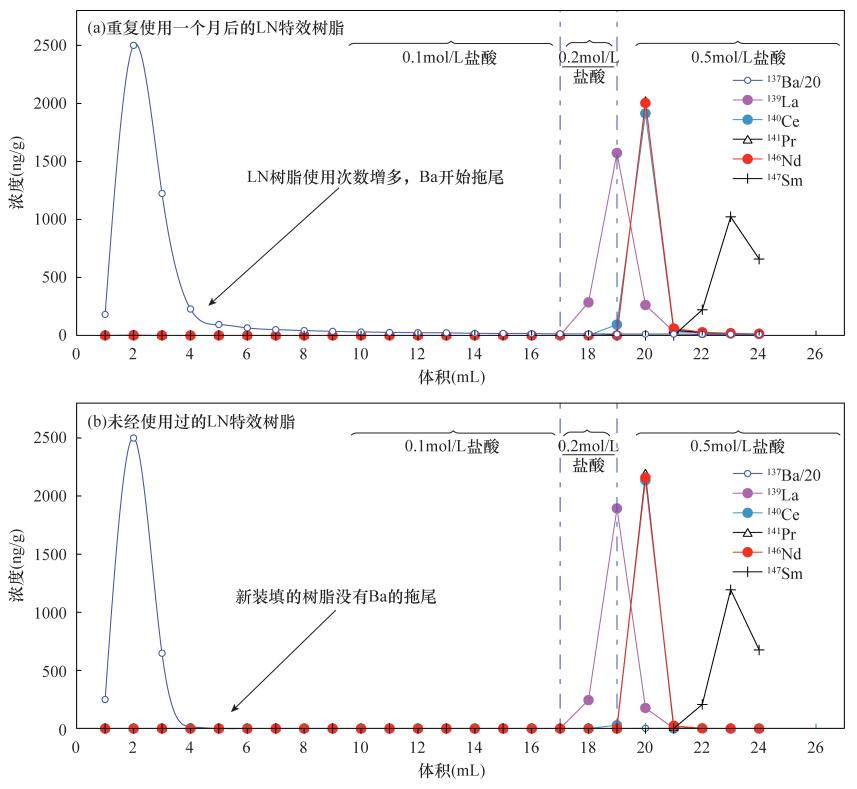
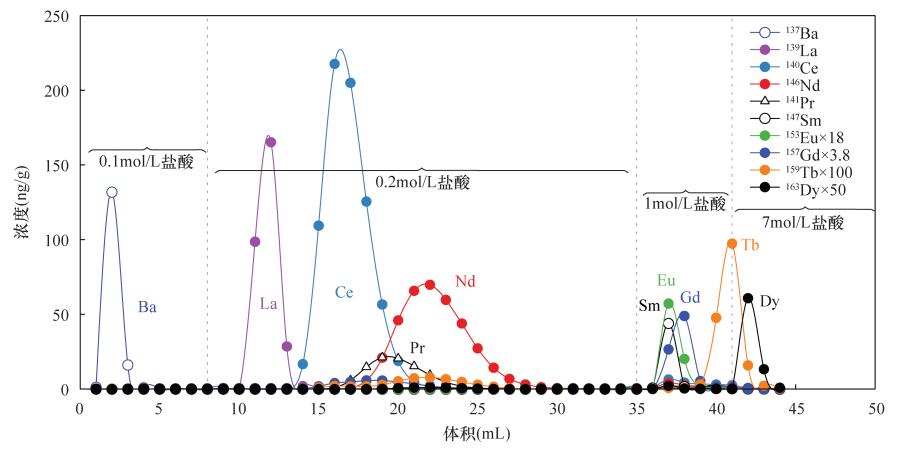
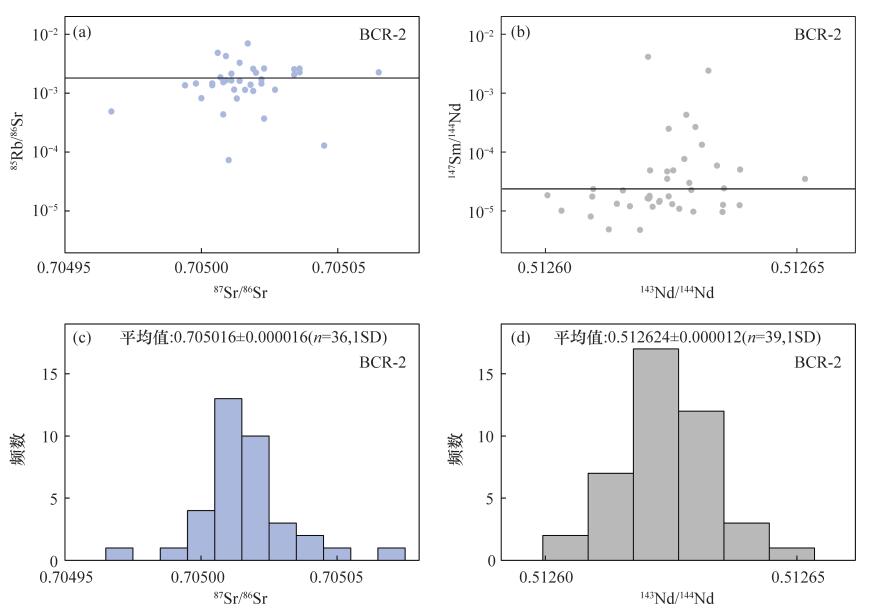
| [1] |
Fisher C, Bauer A, Vervoort J.Disturbances in the Sm-Nd isotope system of the Acasta Gneiss Complex-Implications for the Nd isotope record of the early Earth[J].Earth and Planetary Science Letters, 2020, 530:115900. doi: 10.1016/j.epsl.2019.115900
CrossRef Google Scholar
|
| [2] |
Tillberg M, Drake H, Zack T, Kooijman E, et al.In situ Rb-Sr dating of slicken fibres in deep crystalline basement faults[J]. Scientific Reports, 2020, 10(1):1-13. doi: 10.1038/s41598-019-56847-4
CrossRef Google Scholar
|
| [3] |
Pin C, Gannoun A, Dupont A.Rapid, simultaneous separation of Sr, Pb, and Nd by extraction chromatography prior to isotope ratios determination by TIMS and MC-ICP-MS[J].Journal of Analytical Atomic Spectrometry, 2014, 29(10):1858-1870. doi: 10.1039/C4JA00169A
CrossRef Google Scholar
|
| [4] |
Wang M, Audi G, Kondev F G, et al.The AME2016 atomic mass evaluation (Ⅱ).Tables, graphs and references[J].Chinese Physics C, 2017, 41(3):030003. doi: 10.1088/1674-1137/41/3/030003
CrossRef Google Scholar
|
| [5] |
Vanhaecke F, Degryse P.Isotopic analysis[M].Weinheim:Wiley-VCH Verlag GmbH & Co.KGaA, 2012.
Google Scholar
|
| [6] |
Makishima A.Thermal ionization mass spectrometry (TIMS):Silicate digestion, separation, and measurement[M].New Jersey:John Wiley & Sons, 2016.
Google Scholar
|
| [7] |
Egli D, Müller W, Mancktelow N.Laser-cut Rb-Sr microsampling dating of deformational events in the Mont Blanc-Aiguilles Rouges region (European Alps)[J].Terra Nova, 2016, 28(1):35-42. doi: 10.1111/ter.12184
CrossRef Google Scholar
|
| [8] |
Bolea-Fernandez E, van Malderen S J M, Balcaen L, et al.Laser ablation-tandem ICP-mass spectrometry (LA-ICP-MS/MS) for direct Sr isotopic analysis of solid samples with high Rb/Sr ratios[J].Journal of Analytical Atomic Spectrometry, 2016, 31(2):464-472. doi: 10.1039/C5JA00404G
CrossRef Google Scholar
|
| [9] |
Hogmalm K J, Zack T, Karlsson A K O, et al.In situ Rb-Sr and K-Ca dating by LA-ICP-MS/MS:An evaluation of N2O and SF6 as reaction gases[J].Journal of Analytical Atomic Spectrometry, 2017, 32(2):305-313. doi: 10.1039/C6JA00362A
CrossRef Google Scholar
|
| [10] |
Zack T, Hogmalm K J.Laser ablation Rb/Sr dating by online chemical separation of Rb and Sr in an oxygen-filled reaction cell[J].Chemical Geology, 2016, 437:120-133. doi: 10.1016/j.chemgeo.2016.05.027
CrossRef Google Scholar
|
| [11] |
Korkisch J.Handbook of ion exchange resins:Their application to inorganic analytical chemistry (Volume Ⅵ)[M].Boca Raton:CRC Press, 1989.
Google Scholar
|
| [12] |
濮魏, 高剑锋, 凌洪飞, 等.利用DCTA和HIBA快速有效分离Rb-Sr、Sm-Nd的方法[J].南京大学学报(自然科学版), 2005, 41(4):445-450.
Google Scholar
Pu W, Gao J F, Ling H F, et al.Separation method of Rb-Sr, Sm-Nd using DCTA and HIBA[J].Journal of Nanjing University (Natural Sciences), 2005, 41(4):445-450.
Google Scholar
|
| [13] |
Strelow F W E.Distribution coefficients and ion exchange behavior of 46 elements with a macroreticular cation exchange resin in hydrochloric acid[J].Analytical Chemistry, 1984, 56(6):1053-1056. doi: 10.1021/ac00270a045
CrossRef Google Scholar
|
| [14] |
Strelow F W E.An ion exchange selectivity scale of cations based on equilibrium distribution coefficients[J].Analytical Chemistry, 1960, 32(9):1185-1188. doi: 10.1021/ac60165a042
CrossRef Google Scholar
|
| [15] |
Strelow F W E, Rethemeyer R, Bothma C J C.Ion exchange selectivity scales for cations in nitric acid and sulfuric acid media with a sulfonated polystyrene resin[J].Analytical Chemistry, 1965, 37(1):106-111.
Google Scholar
|
| [16] |
Li C F, Li X H, Li Q L, et al.Rapid and precise deter-mination of Sr and Nd isotopic ratios in geological samples from the same filament loading by thermal ionization mass spectrometry employing a single-step separation scheme[J]. Analytica Chimica Acta, 2012, 727:54-60. doi: 10.1016/j.aca.2012.03.040
CrossRef Google Scholar
|
| [17] |
Li C F, Chu Z Y, Guo J H, et al.A rapid single column separation scheme for high-precision Sr-Nd-Pb isotopic analysis in geological samples using thermal ionization mass spectrometry[J].Analytical Methods, 2015, 7(11):4793-4802. doi: 10.1039/C4AY02896A
CrossRef Google Scholar
|
| [18] |
尹鹏, 何倩, 何会军, 等.离子交换树脂法分离沉积物中锶和钕的影响因素研究[J].岩矿测试, 2018, 37(4):379-387.
Google Scholar
Yin P, He Q, He H J, et al.Study on the factors influencing the separation of Sr and Nd in sediments by ion exchange resin[J].Rock and Mineral Analysis, 2018, 37(4):379-387.
Google Scholar
|
| [19] |
韦刚健, 刘颖, 涂湘林, 等.利用选择性特效树脂富集分离岩石样品中的锶钐和钕[J].岩矿测试, 2004, 23(1):11-14.
Google Scholar
Wei G J, Liu Y, Tu X L, et al.Separation of Sr, Sm and Nd in mineral and rock samples using selective specific resins[J].Rock and Mineral Analysis, 2004, 23(1):11-14.
Google Scholar
|
| [20] |
Glennon K J, Osborn J M, Burns J D, et al.Measuring key Sm isotope ratios in irradiated UO2 for use in plutonium discrimination nuclear forensics[J].Journal of Radioanalytical and Nuclear Chemistry, 2019, 320(2):405-414. doi: 10.1007/s10967-019-06486-w
CrossRef Google Scholar
|
| [21] |
Zhu Z Y, Yang T, Zhu X K.Achieving rapid analysis of Li isotopes in high-matrix and low-Li samples with MC-ICP-MS:New developments in sample preparation and mass bias behavior of Li in ICPMS[J].Journal of Analytical Atomic Spectrometry, 2019, 34(7):1503-1513. doi: 10.1039/C9JA00076C
CrossRef Google Scholar
|
| [22] |
朱志勇, 朱祥坤, 杨涛.自动分离提纯系统的研制及其在同位素分析测试中的应用[J].岩矿测试, 2020, 39(3):384-390.
Google Scholar
Zhu Z Y, Zhu X K, Yang T.A fully automated chemical separation and purification system and its application to isotope analysis[J].Rock and Mineral Analysis, 2020, 39(3):384-390.
Google Scholar
|
| [23] |
Woodhead J, Swearer S, Hergt J, et al.In situ Sr-isotope analysis of carbonates by LA-MC-ICP-MS:Interference corrections, high spatial resolution and an example from otolith studies[J].Journal of Analytical Atomic Spectrometry, 2005, 20(1):22-27. doi: 10.1039/b412730g
CrossRef Google Scholar
|
| [24] |
Yang Y H, Wu F Y, Wilde S A, et al.In situ perovskite Sr-Nd isotopic constraints on the petrogenesis of the Ordovician Mengyin kimberlites in the North China Craton[J].Chemical Geology, 2009, 264(1-4):24-42. doi: 10.1016/j.chemgeo.2009.02.011
CrossRef Google Scholar
|
| [25] |
Davidson J, Tepley Ⅲ F, Palacz Z, et al.Magma recharge, contamination and residence times revealed by in situ laser ablation isotopic analysis of feldspar in volcanic rocks[J].Earth and Planetary Science Letters, 2001, 184(2):427-442.
Google Scholar
|
| [26] |
Khawassek Y M, Eliwa A A, El Sayed A H, et al.Adsorption of rare earth elements by strong acid cation exchange resin thermodynamics, characteristics and kinetics[J]. Applied Sciences, 2019, 1(1):51.
Google Scholar
|
| [27] |
Zawisza B, Pytlakowska K, Feist B, et al.Determination of rare earth elements by spectroscopic techniques:A review[J].Journal of Analytical Atomic Spectrometry, 2011, 26(12):2373-2390. doi: 10.1039/c1ja10140d
CrossRef Google Scholar
|
| [28] |
Li W, Jin X, Gao B, et al.Analysis of ultra-low level rare earth elements in magnetite samples from banded iron formations using HR-ICP-MS after chemical separation[J].Analytical Methods, 2014, 6(15):6125-6132. doi: 10.1039/C4AY00685B
CrossRef Google Scholar
|
| [29] |
Chu Z, Chen F, Yang Y, et al.Precise determination of Sm, Nd concentrations and Nd isotopic compositions at the nanogram level in geological samples by thermal ionization mass spectrometry[J].Journal of Analytical Atomic Spectrometry, 2009, 24(11):1534-1544. doi: 10.1039/b904047a
CrossRef Google Scholar
|
| [30] |
唐索寒, 李津, 梁细荣, 等.钕同位素比值143Nd/144Nd标准溶液研制[J].岩矿测试, 2017, 36(2):163-170.
Google Scholar
Tang S H, Li J, Liang X R, et al.Reference material preparation of 143Nd/144Nd isotope ratio[J].Rock and Mineral Analysis, 2017, 36(2):163-170.
Google Scholar
|
| [31] |
Li C F, Wang X C, Li Y L, et al.Ce-Nd separation by solid-phase micro-extraction and its application to high-precision 142Nd/144Nd measurements using TIMS in geological materials[J].Journal of Analytical Atomic Spectrometry, 2015, 30(4):895-902. doi: 10.1039/C4JA00328D
CrossRef Google Scholar
|
| [32] |
Pin C, Gannoun A.A triple tandem columns extraction chromatography method for isolation of highly purified neodymium prior to 143Nd/144Nd and 142Nd/144Nd isotope ratios determinations[J].Journal of Analytical Atomic Spectrometry, 2019, 34(2):310-318. doi: 10.1039/C8JA00360B
CrossRef Google Scholar
|
| [33] |
Caro G, Bourdon B, Birck J L, et al.High-precision 142Nd/144Nd measurements in terrestrial rocks:Con-straints on the early differentiation of the Earth's mantle[J].Geochimica et Cosmochimica Acta, 2006, 70(1):164-191. doi: 10.1016/j.gca.2005.08.015
CrossRef Google Scholar
|
| [34] |
Upadhyay D, Scherer E E, Mezger K.Fractionation and mixing of Nd isotopes during thermal ionization mass spectrometry:Implications for high precision 142Nd/144Nd analyses[J].Journal of Analytical Atomic Spectrometry, 2008, 23(4):561-568. doi: 10.1039/b715585a
CrossRef Google Scholar
|
| [35] |
Rudnick R L, Gao S.Composition of the continental crust[J].Treatise on Geochemistry, 2003, 3:659.
Google Scholar
|
| [36] |
Lei H L, Yang T, Jiang S Y, et al.A simple two-stage column chromatographic separation scheme for strontium, lead, neodymium and hafnium isotope analyses in geological samples by thermal ionization mass spectrometry or multi-collector inductively coupled plasma mass spectrometry[J].Journal of Separation Science, 2019, 42(20):3261-3275. doi: 10.1002/jssc.201900579
CrossRef Google Scholar
|
| [37] |
Jweda J, Bolge L, Class C, et al.High precision Sr-Nd-Hf-Pb isotopic compositions of USGS reference material BCR-2[J].Geostandards and Geoanalytical Research, 2016, 40(1):101-115. doi: 10.1111/j.1751-908X.2015.00342.x
CrossRef Google Scholar
|
| [38] |
Weis D, Kieffer B, Maerschalk C, et al.High-precision isotopic characterization of USGS reference materials by TIMS and MC-ICP-MS[J].Geochemistry, Geophysics, Geosystems, 2006, 7(8):1-30.
Google Scholar
|





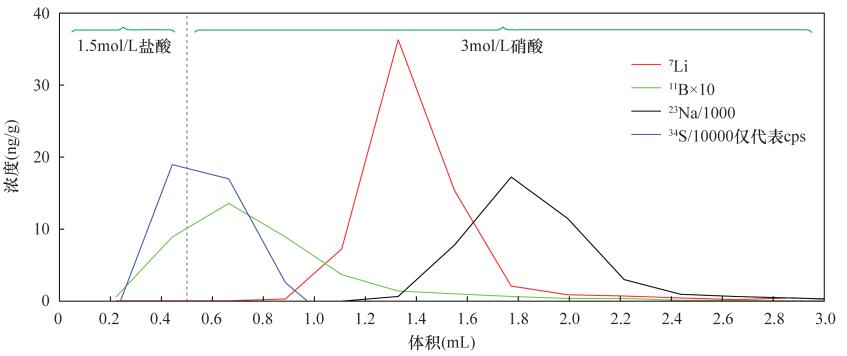

 DownLoad:
DownLoad: