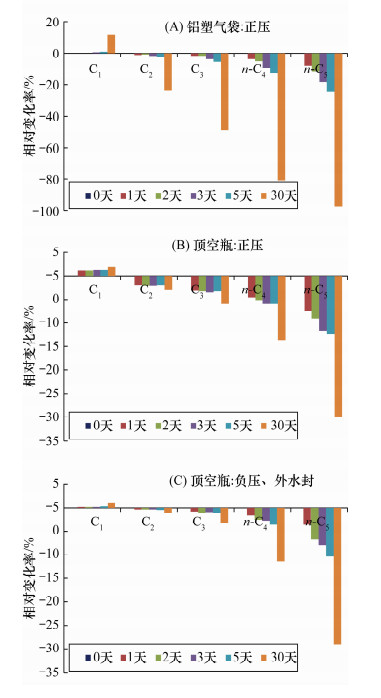
| [1] |
Sloan E D, Koh C A. Clathrate Hydrates of Natural Gases[M]. 3rd Edition. Boca Raton: CRC Press, 2007.
Google Scholar
|
| [2] |
赵祖斌,杨木壮,沙志彬.天然气水合物气体成因及其来源[J].海洋地质动态, 2001, 17(7): 38-41.
Google Scholar
|
| [3] |
孟庆国.海洋天然气水合物模拟实验研究[D].青岛:青岛大学, 2009.
Google Scholar
|
| [4] |
张凌,宁伏龙,蒋国盛,吴翔,窦斌,涂运中.海洋水合物钻探取心与处理现状分析[J].探矿工程(岩土钻掘工程), 2009(Z1): 100-103. doi: 10.3969/j.issn.1672-7428.2009.z1.023
CrossRef Google Scholar
|
| [5] |
张凌,蒋国盛,宁伏龙,吴翔,窦斌,涂运中.国外天然气水合物岩心处理分析技术综述[J].地质科技情报, 2009,28(1): 123-126.
Google Scholar
|
| [6] |
Gudmundsson J S, Parlaktuna M. Storage of Natural Gas Hydrate at Refrigerated Conditons [C]//Proceedings of AICHE Spring National Meeting,1992: 27-32.
Google Scholar
|
| [7] |
Milkov A V. Molecular and stable isotope compositions of natural gas hydrates: A revised global dataset and basic interpretations in the context of geological settings [J]. Organic Geochemistry, 2005,36(5): 681-702. doi: 10.1016/j.orggeochem.2005.01.010
CrossRef Google Scholar
|
| [8] |
Stern L A, Lorenson T D, Pinkston J C. Gas hydrate characterization and grain-scale imaging of recovered cores from the Mount Elbert gas hydrate stratigraphic test well, Alaska North Slope [J]. Marine and Petroleum Geology, 2009, doi:10.1016/j.marpetgeo.
CrossRef Google Scholar
|
| [9] |
Lorenson T D, Collett T S, Hunter R B.Gas geochemistry of the Mount Elbert gas hydrate stratigraphic test well, Alaska North Slope[J]. Marine and Petroleum Geology, 2010. doi:10.1016/j.marpetgeo.2010.02.007 .
CrossRef Google Scholar
|
| [10] |
Vaular E N, Barth T, Haflidason H. The geochemical characteristics of the hydrate-bound gases from the Nyegga pockmark field, Norwegian Sea[J]. Organic Geochemistry, 2010,41(5): 437-444.
Google Scholar
|
| [11] |
Charlou J L, Fouquet Y, Donval J P, Auzende J M, Baptiste P J, Stievenard M, Michel S. Mineral and gas chemistry of hydrothermal fluids on an ultrafast spreading ridge: East Pacific Rise, 17° to 19° (Naudur cruise, 1993)—phase separation processes controlled by volcanic and tectonic activity [J].Journal of Geophysical Research, 1996, 101: 15899-15919. doi: 10.1029/96JB00880
CrossRef Google Scholar
|
| [12] |
贺行良,刘昌岭,孟庆国,祝有海,业渝光,夏宁.祁连山冻土区天然气水合物气体组分的气相色谱法测定[J].地质通报,2011, 30(12): 7-12.
Google Scholar
|
| [13] |
卢振权,祝有海,张永勤,文怀军,李永红,贾志耀,王平康,李清海.青海祁连山冻土区天然气水合物的气体成因研究[J].现代地质,2010,24(3): 581-588.
Google Scholar
|
| [14] |
Pape T, Bahr A, Rethemeyer J, Kessler J D, Sahling H, Hinrichs K U, Klapp S A, Reeburgh W S, Bohrmann G. Molecular and isotopic partitioning of low-molecular-weight hydrocarbons during migration and gas hydrate precipitation in deposits of a high-flux seepage site [J]. Chemical Geology, 2010,269(3-4): 350-363. doi: 10.1016/j.chemgeo.2009.10.009
CrossRef Google Scholar
|
| [15] |
Milkov A V, Claypool G E, Lee Y J, Bohrmann G, Borowski W S, Tomaru H. Gas hydrate systems at Hydrate Ridge Offshore Oregon inferred from molecular and isotopic properties of hydrate-bound and void gases[J].Geochimica et Cosmochimica Acta,2005,69(4): 1007-1026. doi: 10.1016/j.gca.2004.08.021
CrossRef Google Scholar
|
| [16] |
业渝光,刘昌岭,贺行良,孟庆国,孙始财.水合物相平衡原位监测实验装置[P].中国:ZL 2011-2-0099338.9 [2011-01-07].
Google Scholar
|
| [17] |
孟庆国,刘昌岭,业渝光,陈强.不同体系中甲烷水合物储气特性实验研究[J].世界科技研究与发展,2011, 33(1): 25-28.
Google Scholar
|
| [18] |
贺行良,夏宁,刘昌岭,王江涛,孟庆国.FID/TCD并联气相色谱法测定天然气水合物的气体组成[J].分析测试学报,2012,31(2): 206-210.
Google Scholar
|
| [19] |
刘光启,马连湘,刘杰.化学化工物性数据手册(有机卷、无机卷)[M].北京:化学工业出版社,2002.
Google Scholar
|






 DownLoad:
DownLoad: