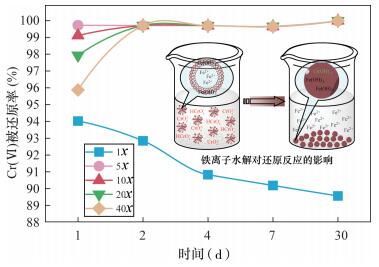
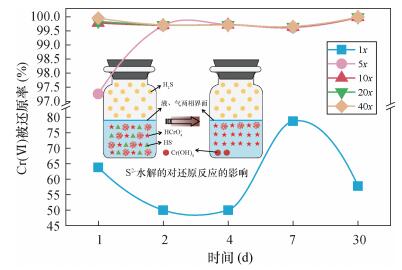
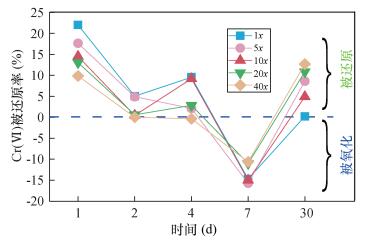
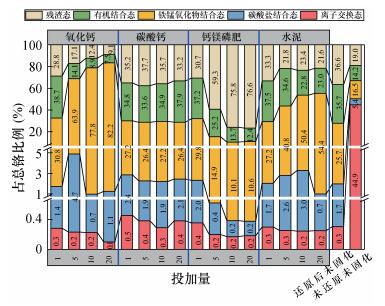
| [1] |
Tardif S, Cipullo S, SøH U, et al. Factors governing the solid phase distribution of Cr, Cu and As in contaminated soil after 40 years of ageing[J].Science of the Total Environment, 2019, 652:744-754. doi: 10.1016/j.scitotenv.2018.10.244
CrossRef Google Scholar
|
| [2] |
田衎, 杨珺, 孙自杰, 等.矿区污染场地土壤重金属元素分析标准样品的研制[J].岩矿测试, 2017, 36(1):82-88.
Google Scholar
Tian K, Yang J, Sun Z J, et al.Preparation of soil certified reference materials for heavy metals in contaminated sites[J].Rock and Mineral Analysis, 2017, 36(1):82-88.
Google Scholar
|
| [3] |
Lü J S, Wang Y M.Multi-scale analysis of heavy metals sources in soils of Jiangsu Coast, Eastern China[J].Chemosphere, 2018, 212:964-973. doi: 10.1016/j.chemosphere.2018.08.155
CrossRef Google Scholar
|
| [4] |
Ma R, Zhou X N, Shi J S.Heavy metal contamination and health risk assessment in critical zone of Luan River catchment in the North China Plain[J].Geochemistry:Exploration, Environment, Analysis, 2018, 18:47-57. doi: 10.1144/geochem2017-010
CrossRef Google Scholar
|
| [5] |
Wang Q, Liu J F, Chen Z, et al.A causation-based method developed for an integrated risk assessment of heavy metals in soil[J].Science of the Total Environment, 2018, 642:1396-1405. doi: 10.1016/j.scitotenv.2018.06.118
CrossRef Google Scholar
|
| [6] |
Diao Z H, Du J J, Jiang D, et al. Insights into the simultaneous removal of Cr6+ and Pb2+ by a novel sewage sludge-derived biochar immobilized nanoscale zero valent iron:Coexistence effect and mechanism[J].Science of the Total Environment, 2018, 642:505-515. doi: 10.1016/j.scitotenv.2018.06.093
CrossRef Google Scholar
|
| [7] |
邓日欣, 罗伟嘉, 韩奕彤, 等.膨润土负载纳米铁镍同步修复地下水中三氯乙烯和六价铬复合污染[J].岩矿测试, 2018, 37(5):541-548.
Google Scholar
Deng R X, Luo W J, Han Y T, et al.Simultaneous removal of TCE and Cr(Ⅵ) in groundwater by using bentonite-supported nanoscale Fe/Ni[J].Rock and Mineral Analysis, 2018, 37(5):541-548.
Google Scholar
|
| [8] |
Economou-Eliopoulos M, Megremi I, Vasilatos C.Geochemical constraints on the sources of Cr(Ⅵ) contamination in waters of Messapia (Central Evia) Basin[J].Applied Geochemistry, 2017, 84:13-25. doi: 10.1016/j.apgeochem.2017.05.015
CrossRef Google Scholar
|
| [9] |
Séby F, Vacchina V. Critical assessment of hexavalent chromium species from different solid environmental, industrial and food matrices[J].Trends in Analytical Chemistry, 2018, 104:54-68. doi: 10.1016/j.trac.2017.11.019
CrossRef Google Scholar
|
| [10] |
Clemention M, Shi X L, Zhang Z.Oxidative stress and metabolic reprogramming in Cr(Ⅵ) carcinogenesis[J].Current Opinion in Toxicology, 2018, 8:20-27. doi: 10.1016/j.cotox.2017.11.015
CrossRef Google Scholar
|
| [11] |
Khalid S, Shahid M, Niazi N K, et al.A comparison of technologies for remediation of heavy metal contaminated soils[J].Journal of Geochemical Exploration, 2017, 182:247-268. doi: 10.1016/j.gexplo.2016.11.021
CrossRef Google Scholar
|
| [12] |
陈保冬, 张莘, 伍松林, 等.丛枝菌根影响土壤-植物系统中重金属迁移转化和累积过程的机制及其生态应用[J].岩矿测试, 2019, 38(1):1-25.
Google Scholar
Chen B D, Zhang X, Wu S L, et al.The role of arbuscular mycorrhizal fungi in heavy metal translocation, transformation and accumulation in the soil-plant continuum:Underlying mechanisms and ecological implications[J].Rock and Mineral Analysis, 2019, 38(1):1-25.
Google Scholar
|
| [13] |
Liu L W, Li W, Song W P, et al.Remediation techniques for heavy metal-contaminated soils:Principles and applicability[J].Science of the Total Environment, 2018, 633:206-219. doi: 10.1016/j.scitotenv.2018.03.161
CrossRef Google Scholar
|
| [14] |
Choppala G, Kunhikrishnan A, Seshadri B, et al.Compa-rative sorption of chromium species as influenced by pH, surface charge and organic matter content in contaminated soils[J].Journal of Geochemical Exploration, 2018, 184:255-260. doi: 10.1016/j.gexplo.2016.07.012
CrossRef Google Scholar
|
| [15] |
Zhang M T, Yang C H, Zhao M, et al.Immobilization potential of Cr(Ⅵ) in sodium hydroxide activated slag pastes[J].Journal of Hazardous Materials, 2017, 321:281-289. doi: 10.1016/j.jhazmat.2016.09.019
CrossRef Google Scholar
|
| [16] |
Ballesteros S, Rincón J M, Rincón-Mora B, et al.Vitrifi-cation of urban soil contamination by hexavalent chromium[J].Journal of Geochemical Exploration, 2017, 174:132-139. doi: 10.1016/j.gexplo.2016.07.011
CrossRef Google Scholar
|
| [17] |
Wu J N, Zhang J, Xiao C Z.Focus on factors affecting pH, flow of Cr and transformation between Cr(Ⅵ) and Cr(Ⅲ) in the soil with different electrolytes[J].Electrochimica Acta, 2016, 211:652-662. doi: 10.1016/j.electacta.2016.06.048
CrossRef Google Scholar
|
| [18] |
Dhal B, Thatoi H N, Das N N, et al.Chemical and micro-bial remediation of hexavalent chromium from contaminated soil and mining/metallurgical solid waste:A review[J].Journal of Hazardous Materials, 2013, 250-251:272-291.
Google Scholar
|
| [19] |
Jiang B, He H H, Liu Y J, et al.pH-dependent roles of polycarboxylates in electron transfer between Cr(Ⅵ) and weak electron donors[J].Chemosphere, 2018, 197:367-374. doi: 10.1016/j.chemosphere.2018.01.047
CrossRef Google Scholar
|
| [20] |
Li Y Y, Liang J L, Yang Z H, et al.Reduction and immobilization of hexavalent chromium in chromite ore processing residue using amorphous FeS2[J].Science of the Total Environment, 2019, 658:315-323. doi: 10.1016/j.scitotenv.2018.12.042
CrossRef Google Scholar
|
| [21] |
Li D, Ji G Z, Hu J, et al.Remediation strategy and electrochemistry flushing & reduction technology for real Cr(Ⅵ)-contaminated soils[J].Chemical Engineering Journal, 2018, 334:1281-1288. doi: 10.1016/j.cej.2017.11.074
CrossRef Google Scholar
|
| [22] |
Zhang X H, Liu J, Huang H T, et al.Chromium accu-mulation by the hyperaccumulator plant Leersia hexandra Swartz[J].Chemosphere, 2007, 67:1138-1143. doi: 10.1016/j.chemosphere.2006.11.014
CrossRef Google Scholar
|
| [23] |
Bai Y N, Lu Y Z, Shen N, et al.Investigation of Cr(Ⅵ) reduction potential and mechanism by Caldicellulosiruptor saccharolyticus under glucose fermentation condition[J].Journal of Hazardous Materials, 2018, 344:585-592. doi: 10.1016/j.jhazmat.2017.10.059
CrossRef Google Scholar
|
| [24] |
Zhang Q, Amor K, Galer S J G, et al.Variations of stable isotope fractionation during bacterial chromium reduction processes and their implications[J].Chemical Geology, 2018, 481:155-164. doi: 10.1016/j.chemgeo.2018.02.004
CrossRef Google Scholar
|
| [25] |
赵庆令, 安茂国, 陈洪年, 等.济南市某废弃化工厂区域土壤地球化学特征研究[J].岩矿测试, 2018, 37(2):201-208.
Google Scholar
Zhao Q L, An M G, Chen H N, et al.Research on geochemical characteristics of soil in a chemical industrial factory site in Jinan city[J].Rock and Mineral Analysis, 2018, 37(2):201-208.
Google Scholar
|
| [26] |
李玲, 唐晓声, 李海建.六价铬污染土壤还原稳定修复[J].广东化工, 2016, 43(3):95-96. doi: 10.3969/j.issn.1007-1865.2016.03.049
CrossRef Google Scholar
Li L, Tang X S, Li H J.Reduction and stabilization remediation of hexavalent chromium contaminated soil[J]. Guangdong Chemical Industry, 2016, 43(3):95-96. doi: 10.3969/j.issn.1007-1865.2016.03.049
CrossRef Google Scholar
|
| [27] |
纪柱.含铬的磷酸盐[J].无机盐工业, 2005, 37(8):8-11. doi: 10.3969/j.issn.1006-4990.2005.08.003
CrossRef Google Scholar
Ji Z.Chromium:Containing phosphate[J].Inorganic Chemicals Industry, 2005, 37(8):8-11. doi: 10.3969/j.issn.1006-4990.2005.08.003
CrossRef Google Scholar
|
| [28] |
Gomm J R, Schwenzer B, Morse D E.Textured films of chromium phosphate synthesized by low-temperature vapor diffusion catalysis[J].Solid State Sciences, 2007, 9:429-431. doi: 10.1016/j.solidstatesciences.2007.03.012
CrossRef Google Scholar
|
| [29] |
Li Y Y, Cundy A B, Feng J X, et al.Remediation of hexa-valent chromium contamination in chromite ore processing residue by sodium dithionite and sodium phosphate addition and its mechanism[J].Journal of Environmental Management, 2017, 192:100-106.
Google Scholar
|
| [30] |
Markelova E, Couture R M, Parsona C T, et al.Specia-tion dynamics of oxyanion contaminants (As, Sb, Cr) in argillaceous suspensions during oxic-anoxic cycles[J].Applied Geochemistry, 2018, 91:75-88. doi: 10.1016/j.apgeochem.2017.12.012
CrossRef Google Scholar
|






 DownLoad:
DownLoad: