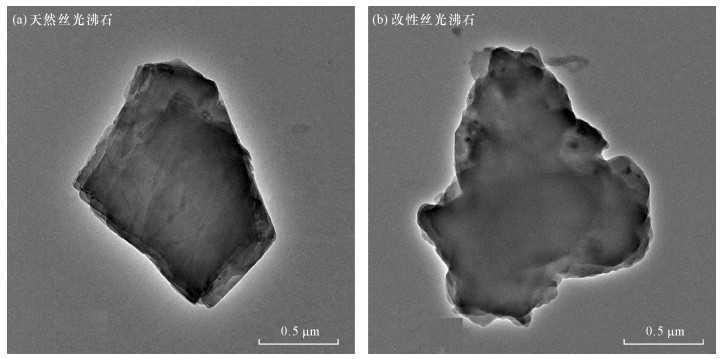
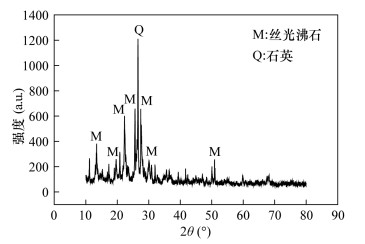
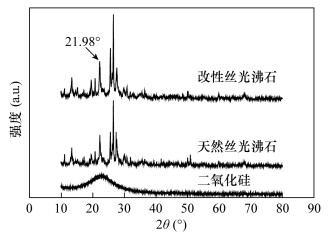
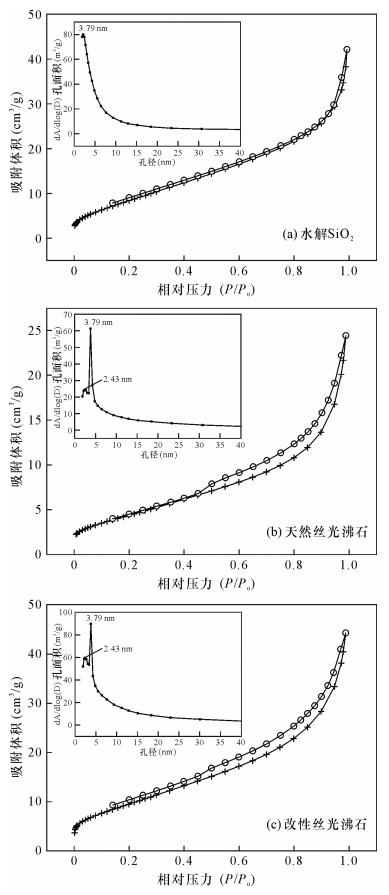
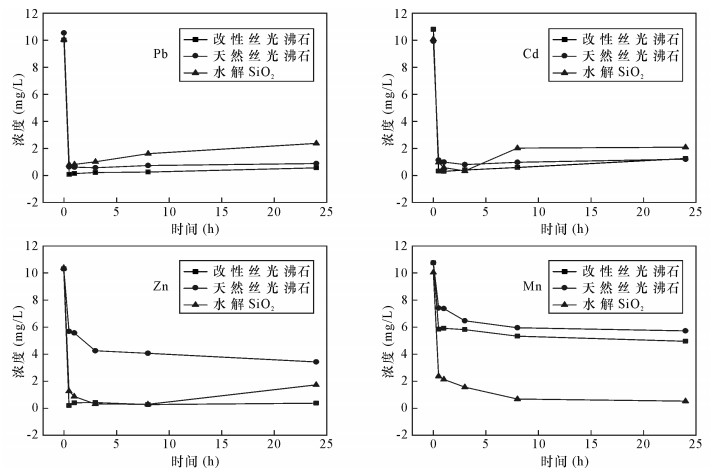
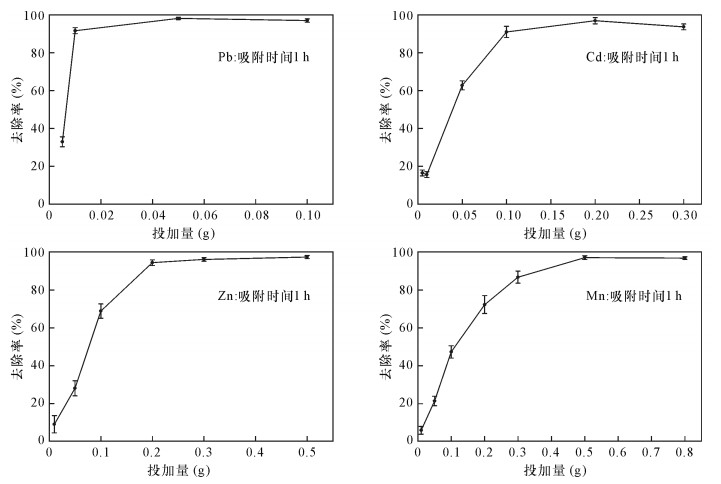
| [1] |
Li Z Y, Ma Z W, van Der Kuijp T J, et al.A review of soil heavy metal pollution from mines in China:Pollution and health risk assessment[J].Science of the Total Environment, 2014, 468:843-853.
Google Scholar
|
| [2] |
Li H X, Ji H B, Shi C J, et al.Distribution of heavy metals and metalloids in bulk and particle size fractions of soils from coal-mine brownfield and implications on human health[J].Chemosphere, 2017, 172:505-515. doi: 10.1016/j.chemosphere.2017.01.021
CrossRef Google Scholar
|
| [3] |
Xu C, Zhou P J, Li H Y, et al.Contamination and spatial distribution of heavy metals in soil of Xiangxi River water-level-fluctuating zone of the Three Gorges Reservoir, China[J].Human and Ecological Risk Assessment, 2017, 23(4):851-863. doi: 10.1080/10807039.2017.1288562
CrossRef Google Scholar
|
| [4] |
Jia Y Y, Wang L, Qu Z P, et al.Distribution, contamin-ation and accumulation of heavy metals in water, sediments, and freshwater shellfish from Liuyang River, Southern China[J].Environmental Science and Pollution Research, 2018, 25(7):7012-7020. doi: 10.1007/s11356-017-1068-x
CrossRef Google Scholar
|
| [5] |
Momodu M A, Anyakora C A.Heavy metal contamination of ground water:The surulere case study[J].Research Journal of Environmental & Earth Sciences, 2010, 2(1):39-43.
Google Scholar
|
| [6] |
Ferati F, Kerolli-Mustafa M, Kraja-Ylli A.Assessment of heavy metal contamination in water and sediments of Trepca and Sitnica rivers, Kosovo, using pollution indicators and multivariate cluster analysis[J].Environmental Monitoring and Assessment, 2015, 187(6):DOI10.1007/s10661-015-4524-4.
Google Scholar
|
| [7] |
Kastyuchik A, Karam A, Aïder M.Effectiveness of alkaline amendments in acid mine drainage remediation[J].Environmental Technology & Innovation, 2016, 6:49-59.
Google Scholar
|
| [8] |
Zhao Y N.Review of the natural, modified, and synthetic zeolites for zeavy metals removal from wastewater[J].Environmental Engineering Science, 2016, 33(7):443-454. doi: 10.1089/ees.2015.0166
CrossRef Google Scholar
|
| [9] |
Son E B, Poo K M, Chang J S, et al.Heavy metal removal from aqueous solutions using engineered magnetic biochars derived from waste marine macro-algal biomass[J].Science of the Total Environment, 2018, 615:161-168. doi: 10.1016/j.scitotenv.2017.09.171
CrossRef Google Scholar
|
| [10] |
Rahman M M, Adil M, Yusof A M, et al.Removal of heavy metal ions with acid activated carbons derived from oil palm and coconut shells[J].Materials (Basel), 2014, 7(5):3634-3650. doi: 10.3390/ma7053634
CrossRef Google Scholar
|
| [11] |
Hizal J, Tutem E, Guclu K, et al.Heavy metal removal from water by red mud and coal fly ash:An integrated adsorption-solidification/stabilization process[J].Desalination and Water Treatment, 2013, 51(37-39):7181-7193. doi: 10.1080/19443994.2013.771289
CrossRef Google Scholar
|
| [12] |
Cao J S, Wang C, Fang F, et al.Removal of heavy metal Cu(Ⅱ) in simulated aquaculture wastewater by modified palygorskite[J].Environmental Pollution, 2016, 219:924-931. doi: 10.1016/j.envpol.2016.09.014
CrossRef Google Scholar
|
| [13] |
谭科艳, 王喆, 蔡敬怡, 等.地球化学工程技术修复江西某Pb超标耕地的应用[J].地球学报, 2017, 38(6):953-960.
Google Scholar
Tan K Y, Wang Z, Cai J Y, et al.The application of geochemical engineering technology to the remediation of a Pb-polluted abandoned farmland in Jiangxi Province[J].Acta Geoscientica Sinica, 2017, 38(6):953-960.
Google Scholar
|
| [14] |
Ciosek A L, Luk G K.Kinetic modelling of the removal of multiple heavy metallic ions from mine waste by natural zeolite sorption[J].Water, 2017, 9(7):DOI:10.3390/w9070482.
CrossRef Google Scholar
|
| [15] |
Inglezakis V J, Fyrillas M M, Stylianou M A.Two-phase homogeneous diffusion model for the fixed bed sorption of heavy metals on natural zeolites[J].Microporous and Mesoporous Materials, 2018, 266:164-176. doi: 10.1016/j.micromeso.2018.02.045
CrossRef Google Scholar
|
| [16] |
Reeve P J, Fallowfield H J.Natural and surfactant modi-fied zeolites:A review of their applications for water remediation with a focus on surfactant desorption and toxicity towards microorganisms[J].Journal of Environmental Management, 2018, 205:253-261.
Google Scholar
|
| [17] |
Wen J, Yi Y J, Zeng G M.Effects of modified zeolite on the removal and stabilization of heavy metals in contaminated lake sediment using BCR sequential extraction[J].Journal of Environmental Management, 2016, 178:63-69. doi: 10.1016/j.jenvman.2016.04.046
CrossRef Google Scholar
|
| [18] |
Mulyati S, Arahman N, Syawaliah, et al.Removal of Me-tal Iron from Groundwater Using Aceh Natural Zeolite and Membrane Filtration[C]//Proceedings of 1st Annual Applied Science and Engineering Conference (Aasec), 2017.
Google Scholar
|
| [19] |
Tran H N, Viet P V, Chao H P.Surfactant modified zeolite as amphiphilic and dual-electronic adsorbent for removal of cationic and oxyanionic metal ions and organic compounds[J].Ecotoxicology and Environmental Safety, 2018, 147:55-63. doi: 10.1016/j.ecoenv.2017.08.027
CrossRef Google Scholar
|
| [20] |
Liu Y, Lou Z M, Sun Y, et al.Influence of complexing agent on the removal of Pb(Ⅱ) from aqueous solutions by modified mesoporous SiO2[J].Microporous and Mesoporous Materials, 2017, 246:1-13. doi: 10.1016/j.micromeso.2017.03.005
CrossRef Google Scholar
|
| [21] |
Zendehdel M, Shoshtari-Yeganeh B, Cruciani G.Removal of heavy metals and bacteria from aqueous solution by novel hydroxyapatite/zeolite nanocomposite, preparation, and characterization[J].Journal of the Iranian Chemical Society, 2016, 13(10):1915-1930. doi: 10.1007/s13738-016-0908-9
CrossRef Google Scholar
|
| [22] |
Kim S A, Kamala-Kannan S, Oh S G, et al.Simultaneous removal of chromium(Ⅵ) and Reactive Black 5 using zeolite supported nano-scale zero-valent iron composite[J].Environmental Earth Sciences, 2016, 75(5):447. doi: 10.1007/s12665-015-4855-z
CrossRef Google Scholar
|
| [23] |
Lin J, Zhan Y.Adsorption of humic acid from aqueous solution onto unmodified and surfactant-modified chitosan/zeolite composites[J].Chemical Engineering Journal, 2012, 200-202:202-213. doi: 10.1016/j.cej.2012.06.039
CrossRef Google Scholar
|
| [24] |
吴昆明, 郭华明, 魏朝俊.改性磁铁矿对水体中砷的吸附特性研究[J].岩矿测试, 2017, 36(6):624-632.
Google Scholar
Wu K M, Guo H M, Wei C J.Adsorption characteristics of arsenic in water by modified magnetite[J].Rock and Mineral Analysis, 2017, 36(6):624-632.
Google Scholar
|
| [25] |
石太宏, 吕灿, 左莉娜.硅烷化改性沸石对重金属离子的吸附性能[J].环境工程学报, 2013, 7(3):1045-1052.
Google Scholar
Shi T H, Lü C, Zuo L N.Adsorption of heavy metal ions by silylation modified zeolite[J].Chinese Journal of Environmental Engineering, 2013, 7(3):1045-1052.
Google Scholar
|
| [26] |
易发成.沸石的活化处理及其对铅、钴的吸附性研究[J].矿物岩石, 2005, 25(3):118-121. doi: 10.3969/j.issn.1001-6872.2005.03.026
CrossRef Google Scholar
Yi F C.The activation treatment and Pb2+, Co2+ ions adsorption capability of zeolite[J].Journal of Mineralogy and Petrology, 2005, 25(3):118-121. doi: 10.3969/j.issn.1001-6872.2005.03.026
CrossRef Google Scholar
|
| [27] |
郝鹏飞, 梁靖, 钟颖.改性沸石对含铅废水的处理研究[J].环境科学与管理, 2009, 34(6):106-108. doi: 10.3969/j.issn.1673-1212.2009.06.031
CrossRef Google Scholar
Hao P F, Liang J, Zhong Y.The research of modified zeolite to the disposal of leaded wastewater[J].Environmental Science and Management, 2009, 34(6):106-108. doi: 10.3969/j.issn.1673-1212.2009.06.031
CrossRef Google Scholar
|
| [28] |
陈溪, 崔天顺, 邓晓军, 等.改性沸石对镉(Ⅱ)的交换性能研究[J].工业安全与环保, 2015, 41(4):55-57. doi: 10.3969/j.issn.1001-425X.2015.04.018
CrossRef Google Scholar
Chen X, Cui T S, Deng X J, et al.Research on the Cd2+ ion exchange by modified zeolite[J].Industrial Safety and Environmental Protection, 2015, 41(4):55-57. doi: 10.3969/j.issn.1001-425X.2015.04.018
CrossRef Google Scholar
|
| [29] |
李蘅, 张静, 徐文炘, 等.沸石处理选矿重金属废水初步研究[J].矿产与地质, 2015, 29(5):682-687. doi: 10.3969/j.issn.1001-5663.2015.05.025
CrossRef Google Scholar
Li H, Zhang J, Xu W X, et al.A preliminary study on treatment of heavy metal beneficiation wastewater by zeolite[J].Mineral Resources and Geology, 2015, 29(5):682-687. doi: 10.3969/j.issn.1001-5663.2015.05.025
CrossRef Google Scholar
|
| [30] |
Maretto M, Bianchi F, Vignola R, et al.Microporous and mesoporous materials for the treatment of wastewater produced by petrochemical activities[J].Journal of Cleaner Production, 2014, 77:22-34. doi: 10.1016/j.jclepro.2013.12.070
CrossRef Google Scholar
|
| [31] |
文叶轩, 郝硕硕, 朱家亮, 等.天然和改性沸石对铬吸附特征研究[J].中国陶瓷, 2015, 51(7):16-20.
Google Scholar
Wen Y X, Hao S S, Zhu J L, et al.Research on adsorption characteristics of chromium on natural and modified zeolite[J].China Ceramics, 2015, 51(7):16-20.
Google Scholar
|





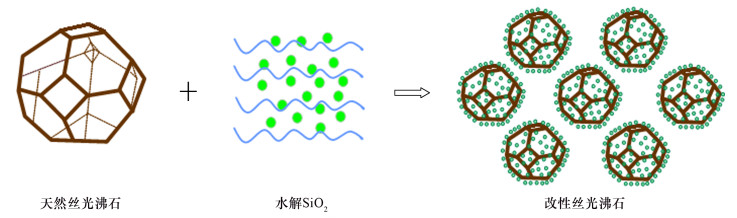

 DownLoad:
DownLoad: