Determination of Major Components in Rock Salt by X-ray Fluorescence Spectrometry with Sample Fusion[J]. Rock and Mineral Analysis, 2016, 35(3): 290-294. doi: 10.15898/j.cnki.11-2131/td.2016.03.012
| Citation: |
Determination of Major Components in Rock Salt by X-ray Fluorescence Spectrometry with Sample Fusion[J]. Rock and Mineral Analysis, 2016, 35(3): 290-294. doi: 10.15898/j.cnki.11-2131/td.2016.03.012
|
Determination of Major Components in Rock Salt by X-ray Fluorescence Spectrometry with Sample Fusion
-
Institute of Multipurpose Utilization of Mineral Resources, Chinese Academy of Geological Sciences, Chengdu 610041, China
-
Abstract
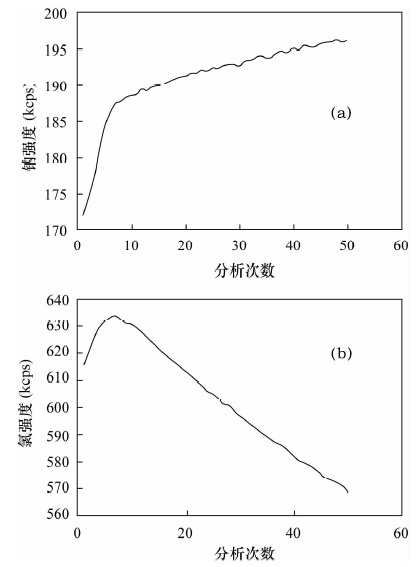
The lack of reference materials, and chlorine loss are two of the problems that need to be solved in order to aid the determination of major components in rock salt by X-ray Fluorescence Spectrometry. It is important to select an appropriate sample pretreatment method to ensure reproducibility and it has been demonstrated that sodium chloride, calcium sulfate and other components in synthetic calibration materials for the pressed pellets method show a tendency of diffusion towards the pellet surface when samples are exposed to X-ray irradiation. Moreover, the decomposition of sodium chloride makes it difficult to obtain a stable calibration curve. The fusion bead method, however, does not exhibit these problems. In the study documented in this paper, samples were prepared by the fusion method. Synthetic calibration materials used for standard curve establishment were prepared by mixing spectrum pure salts/oxides with sediment/soil reference materials. 0.6 g of sample was fused by 10 g of mixing flux of lithium tetraborate and lithium metaborate (12:22) at 1000℃ for 300 s pre-fusion and for another 300 s fusion, followed by 30 s standing. The resulting glass disk was flat and transparent. No extra release agent was added because the samples contained chlorine. Analytical results of the major components in salt rock yielded a precision of less than 1.5% (relative standard deviation). This method shortens the analysis time and reduces the reagent consumption compared with the traditional method, which is an alternative method for analyzing major components in salt rock.
-

-
References
| [1] |
岩石矿物分析编委会.岩石矿物分析(第四版 第二分册)[M].北京:地质出版社,2011:447-449.
Google Scholar
The Editorial Committee of Rock and Mineral Analysis.Rock and Mineral Analysis (Fourth Edition:Vol.Ⅱ) [M].Beijing:Geological Publishing House,2011:447-449.
Google Scholar
|
| [2] |
宋江涛,赵庆令.粉末压片制样-波长色散X射线荧光光谱法测定卤水中的溴[J].岩矿测试,2011,30(4):494-496.
Google Scholar
Song J T,Zhao Q L.Determination of Bromine in Brine Samples by Wavelength Dispersive X-ray Fluorescence Spectrometry with Pressed Powder Preparation[J].Rock and Mineral Analysis,2011,30(4):494-496.
Google Scholar
|
| [3] |
陈景伟,宋江涛,赵庆令,等.薄膜吸附制样-波长色散X射线荧光光谱法测定卤水中的溴[J].岩矿测试,2015,34(5):570-574.
Google Scholar
Chen J W,Song J T,Zhao Q L,et al.Determination of Bromine in Brine by Wavelength Dispersive X-ray Fluorescence Spectrometry with Film Adsorption Pretreatment[J].Rock and Mineral Analysis,2015,34(5):570-574.
Google Scholar
|
| [4] |
Alexander P.X-ray Induced Alteration of Specimens as Crucial Obstacle in XRF Spectrometry of Fluorine in Rocks and Soils[J].X-ray Spectrometry,2013,42(1):19-32.
Google Scholar
|
| [5] |
陆晓明,吉昂,陶光仪.X射线荧光光谱法测定萤石中的氟、钙及二氧化硅[J].分析化学,1997,25(2):178-180.
Google Scholar
Lu X M,Ji A,Tao G Y.Determination of Fluorine,Calcium and Silicon Dioxide in Fluorite by X-ray Fluorescence Spectrometry[J].Chinese Journal of Analytical Chemistry,1997,25(2):178-180.
Google Scholar
|
| [6] |
李可及,肖颖.熔融制样-X射线荧光光谱法测定氟碳铈矿流程样品[J].稀土,2016,37(2):144-148.
Google Scholar
Li K J,Xiao Y.Determination of Bastnaesite Process Samples by Fusion X-ray Fluorescence Spectrometry [J].Chinese Rare Earths,2016,37(2):144-148.
Google Scholar
|
| [7] |
Wiedenbeck M,BédardL P,Bugoi R,et al.GGR Biennial Critical Review:Analytical Developments Since 2012[J].Geostandards and Geoanalytical Research,2014,38(4):467-512.
Google Scholar
|
| [8] |
West M,Ellis A T,Potts P J,et al.2014 Atomic Spectrometry Update-A Review of Advances in X-ray Fluorescence Spectrometry[J].Journal of Analytical Atomic Spectrometry,2014,29:1516-1563.
Google Scholar
|
| [9] |
Classie F,Blanchette J S编著.卓尚军译.硼酸盐熔融的物理和化学[M].上海:华东理工大学出版社,2006:52-61.
Google Scholar
Classie F,Blanchette J S (Editor).Zhuo S J (Translator). Physics and Chemistry of Borate Fusion [M].Shanghai:East China University of Science and Technology Press,2006:52-61.
Google Scholar
|
| [10] |
Nakayama K,Nakamura T.Undersized (12.5mm Diame-ter) Glass Beads with MinimalAmount (11mg) of Geochemical and Archeological Silicic Samples for X-ray Fluorescence Determination of Major Oxides[J].X-Ray Spectrometry,2012,41(4):225-234.
Google Scholar
|
| [11] |
Ichikawa S,Onuma H,Nakamura T.Development of Undersized (12.5mm Diameter) Low-dilution Glass Beads for X-ray Fluorescence Determination of 34 Components in 200mg of Igneous Rock for Applications with Geochemical and Archeological Silicic Samples[J].X-Ray Spectrometry,2016,45(4):34-47.
Google Scholar
|
-
-
Access History







 DownLoad:
DownLoad: