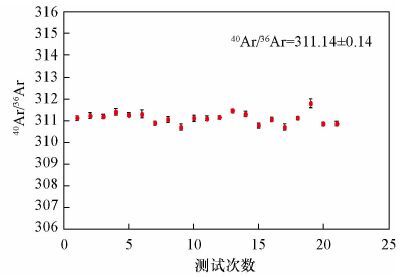
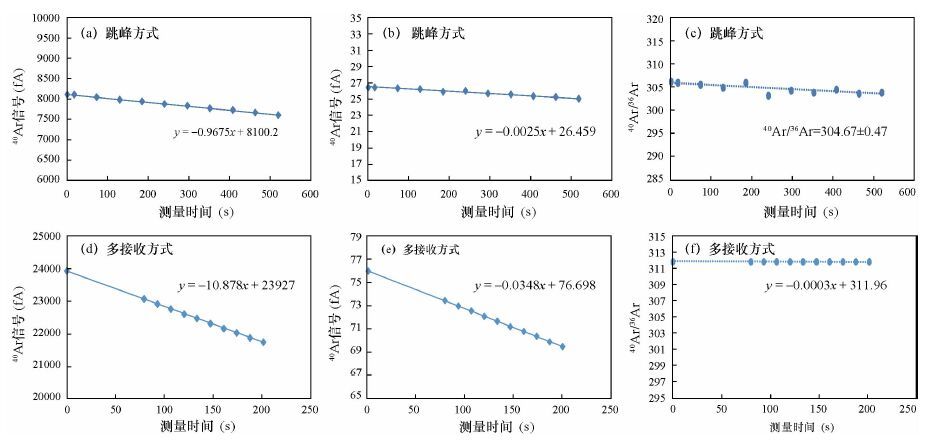
| [1] |
Turrin B D,Swisher C C,Dieno A L.Mass Discrimination Monitor and Intercalibration of Dual Collectors in Noble Gas Mass Spectrometer Systems[J].Geochemistry,Geophysics,Geosystems,2010,11:doi:10.1029/2009GC003013.
CrossRef Google Scholar
|
| [2] |
Mark D F,Stuart F M,de Podesta M.New High Precision Measurement of the Isotopic Composition of Atmospheric Argon[J].Geochimica et Cosmochimica Acta,2011,75:7494-7501.
Google Scholar
|
| [3] |
Renne P R,Sharp W D,Dieno A L,et al.The Isotopic Composition of Atmospheric Argon and Ar/Ar Geochronology:Time for a Change?[J].Quarternary Geochronology,2009,4(4):288-298.
Google Scholar
|
| [4] |
Kim J,Jeon S.40Ar/39 Ar Age Determination Using Argus Ⅵ Multiple-collector Noble Gas Mass Spectrometer:Performance and Its Application to Geosciences[J].Journal of Analytical Science and Technology,2015,6:4-11.
Google Scholar
|
| [5] |
McDougall I,Harrison T M.Geochronology and Thermo-chronology by the 40Ar/39 Ar Method[M].Oxford:Oxford University Press,1999:89-90.
Google Scholar
|
| [6] |
Alexsander E C,Ozima M.Terrestrial Rare Gas[M].Tokyo:Center for Academic Publications,1978.
Google Scholar
|
| [7] |
Mark D F,Barfod D B,Stuart F M,et al.The Argus Multicollector Noble Gas Mass Spectrometer:Performance for Ar/Ar Geochronology[J].Geochemistry,Geophysics,Geosystem,2009,10(2):1-9.
Google Scholar
|
| [8] |
李军杰,李剑,刘汉彬,等.Helix SFT惰性气体质谱仪分析矿物包裹体中氦同位素组成[J].地质学报,2015,89(10):1826-1831.
Google Scholar
Li J J,Li J,Liu H B,et al.The Analysis of Helium Isotope of Inclusions in Minernal Using Helix SFT Noble Gas Mass Spectrometer[J].Acta Geologica Sinica,2015,89(10):1826-1831.
Google Scholar
|
| [9] |
Eiler J M,Clog M, Magyar P,et al.A High-resolution Gas-source Isotope Ratio Mass Spectrometer[J].International Journal of Mass Spectrometry[J].http://dx.doi.org/10.1016/j.ijms.2012.10.014.2012.
Google Scholar
|
| [10] |
Turrin B D,Swisher C C,Deino A L.Mass Discrimination Monitoring and Intercalibration of Dual Collectors in Noble Gas Mass Spectrometer Systems[J].Geochemistry,Geophysics,Geosystem,2010,11.doi:10.1029/2009GC003013.
CrossRef Google Scholar
|
| [11] |
Burnard P G,Farley K A.Calibration of Pressure-dependent Sensitivity and Discrimination in Nier-type Noble Gas Ion Sources[J].Geochemistry,Geophysics,Geosystem,2000,1:33-38.
Google Scholar
|
| [12] |
Baur H A.Noble-gas Mass Spectrometer Compressor Source with Two Orders of Magnitude Improvement in Sensitivity[J].EOS,1999,80:1118.
Google Scholar
|
| [13] |
Reynolds J H.High Sensitivity Mass Spectrometer for Noble Gas Analysis[J].Review of Scientific Instrument,1956,27(11):928-934.
Google Scholar
|
| [14] |
Ryuji O,Tomoki N,Nobuo T,et al.Noble Gases in Ureilites Released by Crushing[J].Meteoritics and Planetary Science,2003,5:767-781.
Google Scholar
|
| [15] |
Ozima M,Podosek F A.Noble Gas Geochemistry[M].Cambridge:Cambridge University Press,2002:286.
Google Scholar
|
| [16] |
Baski A K,Archibald D A,Farrar E.Intercalibration of 40Ar/39 Ar Dating Standards[J].Chemical Geology,1996,129:307-324.
Google Scholar
|
| [17] |
Rudenhauer F G.Gas Scattering as a Limit to Partial-pressure Sensitivity[J].Vacuum Science Technology,1972,9:215.
Google Scholar
|
| [18] |
Lee J Y,Marti K,Severinghaus K,et al.A Redeter-mination of the Isotopic Abundances of Atmospheric Ar[J].Geochimica et Cosmochimica Acta,2006,70:4507-4512.
Google Scholar
|
| [19] |
Holst B,Buckland J R,Allison W.Spatial Mapping in the Electron Impact Ion Source of a Residual Gas Analyser[J].Vacuum,1999,53:207-210.
Google Scholar
|
| [20] |
Nier A O.A Redetermination of the Relative Abundances of the Isotopes of Carbon,Nitrogen,Oxygen,Argon and Potassium[J].Physics Review,1950,77:789-793.
Google Scholar
|
| [21] |
Young E D,Galy A,Nagahara H.Kinetc and Equilibrium Mass-dependent Isotope Fractionation Laws in Nature and Their Geochemical and Cosmochemical Significance[J].Geochmica et Cosmochimica Acta,2002,66(6):1095-1104.
Google Scholar
|
| [22] |
Valkiers S,Vendelbo D,Berglund M,et al.Preparation of Argon Primary Measurement Standards for the Calibration of Ion Current Ratios Measured in Argon[J].International Journal of Mass Spectrometry,2010,291:41-47.
Google Scholar
|





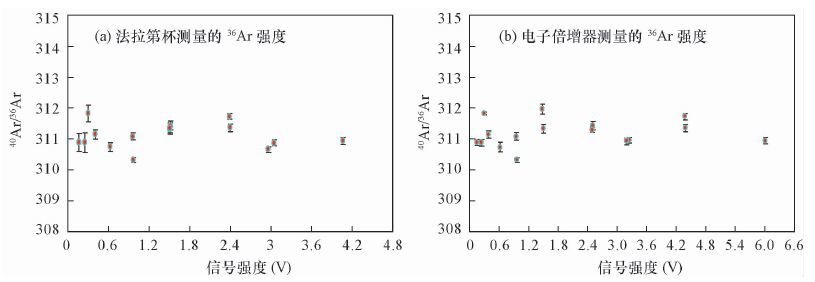

 DownLoad:
DownLoad: