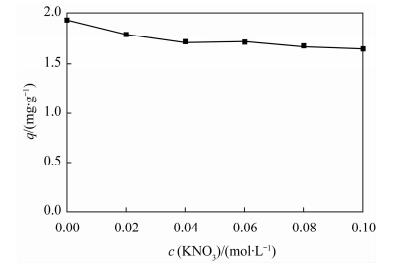
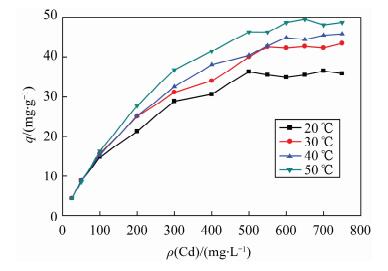
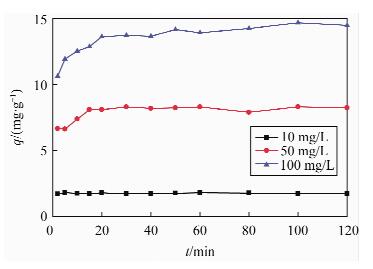
| [1] |
梁彦秋,刘婷婷,铁梅,邓斌,孙鹏,藏树良.镉污染土壤中镉的形态分析及植物修复技术研究[J].环境科学与技术,2007,2(3): 57-58.
Google Scholar
|
| [2] |
鞠建英,申东炫.膨润土在工程中的开发与应用[M].北京: 中国建材工业出版社,2003: 1-2.
Google Scholar
|
| [3] |
Balomenou G, Stathi P, Enotiadis A, Gournis D, Deligian-nakis Y. Physicochemical study of amino-functionalized organosilicon cubes intercalated in montmorillonite clay: H-binding and metal uptake [J]. Journal of Colloid and Interface Science,2008,325: 74-83. doi: 10.1016/j.jcis.2008.04.072
CrossRef Google Scholar
|
| [4] |
原金海,邓利均.改性膨润土的制备及其对Pb2+的吸附性能研究[J].功能材料,2011,42(6): 980-984.
Google Scholar
|
| [5] |
李增新,王彤,黄海兰,张道来,孟韵.壳聚糖改性膨润土修复土壤镉污染的研究[J].土壤通报,2009,40(1): 176-178.
Google Scholar
|
| [6] |
朱霞萍.酸雨条件下珠江三角洲土壤中镉砷的迁移规律研究[D].成都: 成都理工大学, 2008.
Google Scholar
|
| [7] |
李虎杰,刘爱平,易发成,白萍.膨润土对Cd2+的吸附作用及影响因素[J].中国矿业,2004(11): 79-81. doi: 10.3969/j.issn.1004-4051.2004.11.024
CrossRef Google Scholar
|
| [8] |
曾江萍.镉在膨润土中的吸附和解吸行为研究[D].成都: 成都理工大学,2008.
Google Scholar
|
| [9] |
朱霞萍,白德奎,李锡坤,曾江萍,曹三勇.镉在蒙脱石等黏土矿物上的吸附行为研究[J].岩石矿物学杂志,2009,28(6): 643-648.
Google Scholar
|
| [10] |
Huang R H, Wang B, Yang B C, Zheng D S, Zhang Z Q.Equilibrium, kinetic and thermodynamic studies of adsorption of Cd(Ⅱ) from aqueous solution onto HACC-bentonite [J]. Desalination,2011,280: 297-304. doi: 10.1016/j.desal.2011.07.033
CrossRef Google Scholar
|
| [11] |
周建兵,吴平霄,朱能武,党志.十二烷基磺酸钠(SDS)改性蒙脱石对Cu2+、Cd2+的吸附研究[J].环境科学学报,2010,30(1): 88-96.
Google Scholar
|
| [12] |
王毅,王艺,王恩德.改性蒙脱石吸附Pb2+、Hg2+的实验研究[J].岩石矿物学杂志,2001,20(4): 565-567.
Google Scholar
|
| [13] |
张一平.膨润土的巯基功能化研究[J].浙江教育学院学报,2007(1): 35-37.
Google Scholar
|
| [14] |
Mercier L, Preparation C D. Characterization, and application as heavy metals sorbents of covalently grafted thiol functionalities on the interlamellar surface of montmorillonite [J]. Environmental Science & Technology , 1995, 29: 1318-1323.
Google Scholar
|
| [15] |
Malferrari D, Brigatti M F, Laurora A, Pini S, Medici L. Sorption kinetics and chemical forms of Cd (Ⅱ) sorbed by thiol-functionalized 2∶1 clay minerals [J]. Journal of Hazardous Materials, 2007,143: 73-81. doi: 10.1016/j.jhazmat.2006.08.069
CrossRef Google Scholar
|
| [16] |
Liang X F,Xu Y M,Sun G H,Wang L,Sun Y,Qin X.Preparation, characterization of thiol-functionalized silica and application for sorption of Pb2+and Cd2+ [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects,2009,349: 61-68.
Google Scholar
|
| [17] |
李虎杰.盐亭膨润土的物化性能及对Pb2+、Cd2+的吸附研究[J].中国矿业,2006,15(5): 76-79.
Google Scholar
|
| [18] |
Ijagbemi C O, Baek M H, Kim D S.Adsorptive perfor-mance of un-calcined sodium exchanged and acid modified montmorillonite for Ni2+removal: Equilibrium, kinetics, thermodynamics and regeneration studies [J]. Journal of Hazardous Materials, 2010,174: 746-755. doi: 10.1016/j.jhazmat.2009.09.115
CrossRef Google Scholar
|
| [19] |
Eren E.Removal of lead ions by Unye (Turkey) bentonite in iron and magnesium oxide-coated forms [J]. Journal of Hazardous Materials,2009,165: 63-70. doi: 10.1016/j.jhazmat.2008.09.066
CrossRef Google Scholar
|
| [20] |
Grawal A A, Sahu K K. Kinetic and isotherm studies of cadmium adsorption on manganese nodule residue [J]. Journal of Hazardous Materials,2006,137: 915-924. doi: 10.1016/j.jhazmat.2006.03.039
CrossRef Google Scholar
|
| [21] |
Yang S T, Zhao D L, Zhang H, Lu S S, Chen L, Yu X J. Impact of environmental conditions on the sorption behavior of Pb(Ⅱ) in Na-bentonite suspensions [J]. Journal of Hazardous Materials,2010,183: 632-640. doi: 10.1016/j.jhazmat.2010.07.072
CrossRef Google Scholar
|
| [22] |
von Open B, Kordel W, Klein W.Sorption of nonpolar and polar compounds to soils: Processes, measurement and experience with the applicability of the modified OECD-guideline [J]. Chemosphere,1991,22: 285-304. doi: 10.1016/0045-6535(91)90318-8
CrossRef Google Scholar
|
| [23] |
李朝丽,周立祥.黄棕壤不同粒级组分对镉的吸附动力学与热力学研究[J].环境科学,2008,29(5): 1406-1411.
Google Scholar
|





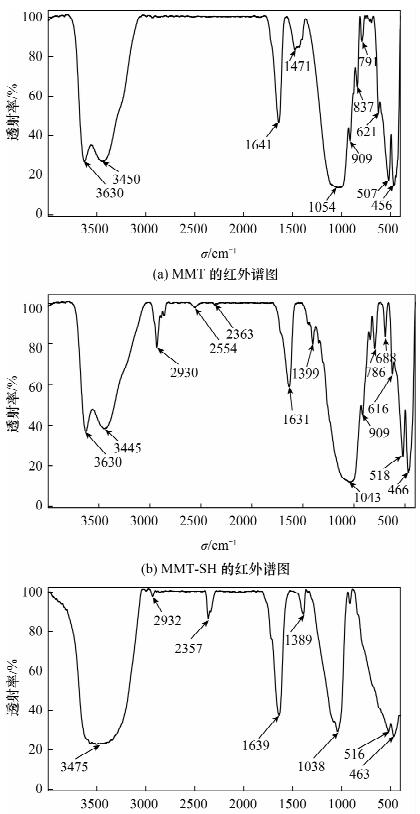

 DownLoad:
DownLoad: