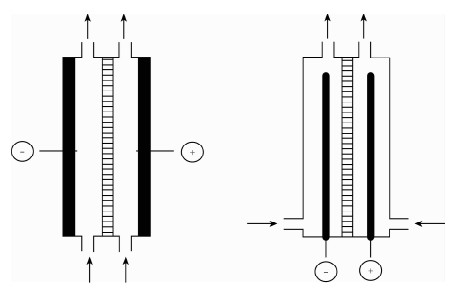
| [1] |
Rie R R, Rikke V H, Erik H L, Jens J S.Development and validation of an SPE HG-AAS method for determination of inorganic arsenic in samples of marine origin[J].Analytical and Bioanalytical Chemistry, 2012, 403(10):2825-2834. doi: 10.1007/s00216-012-6006-7
CrossRef Google Scholar
|
| [2] |
Rauret G, Rubio R, Padró A.Arsenic speciation using HPLC-HG-ICP-AES with gas-liquid separator[J].Fresenius' Journal of Analytical Chemistry, 1991, 340(3):157-160. doi: 10.1007/BF00324472
CrossRef Google Scholar
|
| [3] |
Sayago A, Beltránand R, Josú L, Ariza G. Hydride generation atomic fluorescence spectrometry(HG-AFS) as a sensitive detector for Sb(Ⅲ) and Sb(Ⅴ) speciation in water[J].Journal of Analytical Atomic Spectrometry, 2000,15(4):423-428. doi: 10.1039/A910303L
CrossRef Google Scholar
|
| [4] |
Holak W. Gas-sampling technique for arsenic determi-nation by atomic absorption spectrophotometry[J].Analytical Chemistry, 1969, 41:1712. doi: 10.1021/ac60281a025
CrossRef Google Scholar
|
| [5] |
邓勃,迟锡增,刘明钟,李玉珍.应用原子吸收与原子荧光光谱分析[M].北京:化学工业出版社,2003:38-41.
Google Scholar
|
| [6] |
李淑萍,郭旭明,黄本立,胡荣宗,王秋泉,李彬.电化学氢化物发生法的进展及其在原子光谱分析中的应用[J].分析化学,2001,29(8):967-970.
Google Scholar
|
| [7] |
Francisco L, Eduardo B, Juan R C. Electrochemical hydride generation as a sample-introduction technique in atomic spectrometry: Fundamentals, interferences, and applications[J]. Analytical and Bioanalytical Chemistry, 2007, 388:743-751. doi: 10.1007/s00216-006-1037-6
CrossRef Google Scholar
|
| [8] |
侯逸众,申屠超,范云场,朱岩,陈梅兰.土壤中锑的双阳极电化学氢化物发生-原子荧光光谱法测定[J].分析测试学报,2009,28(1):109-111.
Google Scholar
|
| [9] |
张王兵,淦五二,苏庆德,林祥钦.石墨管阴极电化学氢化物发生原子荧光法测定锗[J].分析化学,2005,33(10):1449-1451. doi: 10.3321/j.issn:0253-3820.2005.10.024
CrossRef Google Scholar
|
| [10] |
张王兵.电化学氢化物发生原子荧光法测定环境样品中的锑[J].应用化学,2009,26(6):738-741.
Google Scholar
|
| [11] |
姜宪娟,淦五二.聚苯胺修饰电极-电化学氢化物发生原子荧光光谱法测定食品中锡含量[J].分析试验室,2012,31(3):101-104.
Google Scholar
|
| [12] |
申屠超,侯逸众,范云场,朱岩.双阳极电化学氢化物发生原子荧光光谱法测定砷[J].分析化学,2008,36(11):1592-1596. doi: 10.3321/j.issn:0253-3820.2008.11.029
CrossRef Google Scholar
|
| [13] |
Li X, Wang Z H. Determination of mercury by intermittent flow electrochemical cold vapor generation coupled to atomic fluorescence spectrometry[J]. Analytica Chimica Acta, 2007, 588(2):179-183. doi: 10.1016/j.aca.2007.02.003
CrossRef Google Scholar
|
| [14] |
淦五二,张王兵,苏庆德.电化学氢化物发生原子荧光法同时测定砷和锑[J].分析化学, 2005, 33(5):687-689.
Google Scholar
|
| [15] |
Zhang W B, Yang X A, Dong Y P, Chu X F. Application of alkaline mode electrochemical hydride generation for the detection of As and Sb using atomic fluorescence spectrometry[J].Spectrochimica Acta Part B: Atomic Spectroscopy, 2010, 65(7):571-578. doi: 10.1016/j.sab.2010.06.003
CrossRef Google Scholar
|
| [16] |
张王兵.电化学氢化物发生.原子荧光法测定环境样品中的Se(Ⅳ)和Se(Ⅵ)[J].分析试验室, 2009,28(5):83-85.
Google Scholar
|
| [17] |
侯逸众,范云场,朱岩,陈梅兰,申屠超.离子色谱-双阳极电化学氢化物发生-原子荧光光谱法测定当归中Sb(Ⅲ)和Sb(Ⅴ)[J].分析试验室,2009, 28(10):38-40. doi: 10.3969/j.issn.1000-0720.2009.10.010
CrossRef Google Scholar
|
| [18] |
申屠超,侯逸众,范云场,朱岩.离子色谱-双阳极电化学氢化物发生-原子荧光光谱法测定Ⅰ型牙髓失活材料中的砷形态[J].分析化学, 2009, 37(2):263-266.
Google Scholar
|
| [19] |
Shen T C, Fan Y C, Hou Y Z, Wang K X, Zhu Y.Arsenic species analysis by ion chromatography-bianode electrochemical hydride generator-atomic fluorescence spectrometry[J].Journal of Chromatography A, 2008,1213(1):56-61. doi: 10.1016/j.chroma.2008.10.016
CrossRef Google Scholar
|
| [20] |
祖文川.电化学冷蒸气发生-原子荧光光谱联用对有机汞的检测研究[D].北京:北京师范大学,2009.
Google Scholar
|
| [21] |
Kozak L, Rudnicka M, Niedzielski P.Determination of inorganic selenium species in dietary supplements by hyphenated analytical system HPLC-HG-AAS[J].Food Analytical Methods, 2012,5(6):1237-1243. doi: 10.1007/s12161-012-9365-y
CrossRef Google Scholar
|
| [22] |
Macedo S M, de Jesus R M, Garcia K S, Hatje V, de Queiroz A F S, Antonio F, Ferreira S L C.Determination of total arsenic and arsenic(Ⅲ) in phosphate fertilizers and phosphate rocks by HG-AAS after multivariate optimization based on Box-Behnken design[J].Talanta, 2009,80(2):974-979. doi: 10.1016/j.talanta.2009.08.025
CrossRef Google Scholar
|
| [23] |
Óscar M N, Raquel D G, Adela B B, José A C, José M F,Pilar B B.Determination of total selenium and selenium distribution in the milk phases in commercial cow's milk by HG-AAS[J].Analytical and Bioanalytical Chemistry, 2005, 381(6):1145-1151. doi: 10.1007/s00216-004-3010-6
CrossRef Google Scholar
|
| [24] |
Jutta F, Michael K, William S.Direct determination of arsenic in acid digests of plant and peat samples using HG-AAS and ICP-SF-MS[J].Analytica Chimica Acta, 2005, 530(2):307-316. doi: 10.1016/j.aca.2004.09.077
CrossRef Google Scholar
|
| [25] |
Schloske L, Waldner H, Marx F.Optimisation of sample pre-treatment in the HG-AAS selenium analysis[J].Analytical and Bioanalytical Chemistry,2002,372(5-6):700-704. doi: 10.1007/s00216-001-1229-z
CrossRef Google Scholar
|
| [26] |
刘文涵,单胜艳,张丹,韩雯雯.流通式电化学氢化物发生法原子吸收测定硒的研究[J].分析测试学报, 2005, 24(5):69-71.
Google Scholar
|
| [27] |
Arbab-Zavara M H, Chamsaza M, Youssefib A, Aliakbari M.Electrochemical hydride generation atomic absorption spectrometry for determination of cadmium[J].Analytica Chimica Acta, 2005,546(1):126-132. doi: 10.1016/j.aca.2005.05.011
CrossRef Google Scholar
|
| [28] |
Sáenz M, Fernández L, Domínguez J, Alvarado J.Electrochemical generation of volatile lead species using a cadmium cathode: Comparison with graphite, glassy carbon and platinum cathodes[J].Spectrochimica Acta Part B: Atomic Spectroscopy,2012, 71-72:107-111. doi: 10.1016/j.sab.2012.03.009
CrossRef Google Scholar
|
| [29] |
Arbab-Zavara M H, Chamsaza M, Youssefib A,Aliakbari M.Flow injection electrochemical hydride generation atomic absorption spectrometry for the determination of cadmium in water samples[J].Microchemical Journal, 2013, 108:188-192. doi: 10.1016/j.microc.2012.10.017
CrossRef Google Scholar
|
| [30] |
Arbab-Zavara M H, Chamsaza M, Youssefib A,Aliakbari M.Multivariate optimization on flow-injection electrochemical hydride generation atomic absorption spectrometry of cadmium[J].Talanta, 2012,97(15):229-234.
Google Scholar
|
| [31] |
Bolea E, Arroyo D, Laborda F, Castillo J R.Determination of antimony by electrochemical hydride generation atomic absorption spectrometry in samples with high iron content using chelating resins as on-line removal system[J]. Analytica Chimica Acta, 2006, 569(1-2):227-233. doi: 10.1016/j.aca.2006.03.076
CrossRef Google Scholar
|
| [32] |
Arbab-Zavar M H, Chamsaza M, Yousefib A, Ashraf N.Electrochemical hydride generation of thallium[J].Talanta, 2009, 79:302-307. doi: 10.1016/j.talanta.2009.03.052
CrossRef Google Scholar
|
| [33] |
Arbab-Zavar M H, Chamsaza M, Yousefib A, Aliakbari M.Evaluation of electrochemical generation of volatile zinc hydride by heated quartz tube atomizer atomic absorption spectrometry[J].Analytical Sciences, 2012, 28:717-722. doi: 10.2116/analsci.28.717
CrossRef Google Scholar
|
| [34] |
Mahboubeh M.Determination of cadmium in environmental sample by electrochemical hydride generation electrothermal atomic absorption with in situ trapping in graphite tube atomizer[J].Research Journal of Pharmaceutical, Biological and Chemical Sciences, 2011, 2(2):910-919.
Google Scholar
|
| [35] |
Mahboubeh M, Raham S. Electrochemical hydride generation of tin (Ⅱ) and its determination by electrothermal atomic absorption spectrometry with in situ trapping in the graphite tube atomizer[J].Toxicological & Environmental Chemistry,2011, 93(7):1332-1340.
Google Scholar
|
| [36] |
Šíma J, Rychlovsky P.Electrochemical selenium hydride generation with in situ trapping in graphite tube atomizers[J].Spectrochimica Acta Part B: Atomic Spectroscopy, 2003,58(5):919-930. doi: 10.1016/S0584-8547(03)00035-1
CrossRef Google Scholar
|
| [37] |
李勋,戚绮,薛珺,朱亚晋.电化学氢化物发生与原子吸收光谱联用对鲜牛奶中无机砷的形态分析[J].食品研究与开发,2007,28(11):121-123. doi: 10.3969/j.issn.1005-6521.2007.11.037
CrossRef Google Scholar
|
| [38] |
李勋,戚绮,薛珺,张晏杰.电化学氢化物发生-原子吸收光谱法测定不同价态的无机砷[J].理化检验(化学分册),2007,43(12):1027-1029.
Google Scholar
|
| [39] |
Li X, Jia J, Wang Z H.Speciation of inorganic arsenic by electrochemical hydride generation atomic absorption spectrometry[J].Analytica Chimica Acta,2006, 560(1-2):153-158. doi: 10.1016/j.aca.2005.12.054
CrossRef Google Scholar
|
| [40] |
Pyell U, Dworschak A, Nitschke F, Neidhart B.Flow injection electrochemical hydride generation atomic absorption spectrometry (FI-EHG-AAS) as a simple device for the speciation of inorganic arsenic and selenium[J].Fresenius' Journal of Analytical Chemistry,1999,363:495-498. doi: 10.1007/s002160051232
CrossRef Google Scholar
|
| [41] |
Denkhaus E, Beck F, Bueschle P, Gerhard R, Golloch A.Electrolytic hydride generation atomic absorption spectrometry for the determination of antimony, arsenic, selenium, and tin-mechanistic aspects and figures of merit[J].Fresenius' Journal of Analytical Chemistry, 2001,370:735-743. doi: 10.1007/s002160100718
CrossRef Google Scholar
|
| [42] |
张丽娟,赵丽巍,刘勤华,李敏晶,陈焕文,张寒琦,金钦汉,赵陆陆.在线分离富集微波等离子体发射光谱法测定Cd,Cu,Zn[J].吉林大学自然科学学报,2000(2):101-103.
Google Scholar
|
| [43] |
李永生,赵博,孙旭辉.流动注射等离子体炬原子发射光谱峰宽定量法[J].光谱学与光谱分析,2009,29(9):2560-2564.
Google Scholar
|
| [44] |
Savio M, Pacheco P H, Martinez L D, Smichowski P, Gil R A.Optimization of methods to assess levels of As, Bi, Sb and Se in airborne particulate matter by FI-HG-ICP-OES[J].Journal of Analytical Atomic Spectrometry, 2010, 25(8):1343-1347. doi: 10.1039/c003864d
CrossRef Google Scholar
|
| [45] |
Tyburska A, Jankowski K, Ramsza A, Reszke E, Strzele M, Andrzejczuk A.Feasibility study of the determination of selenium, antimony and arsenic in drinking and mineral water by ICP-OES using a dual-flow ultrasonic nebulizer and direct hydride generation[J].Journal of Analytical Atomic Spectrometry,2010, 25(2):210-214. doi: 10.1039/B916729C
CrossRef Google Scholar
|
| [46] |
Elena P V, Adela B B, Pilar B B.Use of lanthanum hydroxide as a trapping agent to determine of hydrides by HG-ICP-OES[J].Journal of Analytical Atomic Spectrometry, 2005, 20(12):1344-1349. doi: 10.1039/b509718e
CrossRef Google Scholar
|
| [47] |
Suárez C A, Giné M F. A reactor/phase separatorcoupling capillary electrophoresis to hydride generation and inductively coupled plasma optical emission spectrometry (CE-HG-ICP-OES) for arsenic speciation[J].Journal of Analytical Atomic Spectrometry, 2005, 20(12):1395-1397. doi: 10.1039/b508081a
CrossRef Google Scholar
|
| [48] |
Pohl P, Lesniewicz A, Zyrnicki W.Determination of As, Bi, Sb and Sn in conifer needles from various locations in Poland and Norway by hydride generation inductively coupled plasma atomic emission spectrometry[J].International Journal of Environmental Analytical Chemistry, 2003, 83(11):963-970. doi: 10.1080/03067310310001608731
CrossRef Google Scholar
|
| [49] |
Moyano S, Wuilloud R G, Olsina R A, Gásquez J A, Martinez L D.On-line preconcentration system for bismuth determination in urine by flow injection hydride generation inductively coupled plasma atomic emission spectrometry[J].Talanta, 2001, 54(2):211-219. doi: 10.1016/S0039-9140(01)00310-1
CrossRef Google Scholar
|
| [50] |
Schermer S, Jurica L, Paumard J, Beinrohr E, Matysik F M, Broekaert J A C.Optimization of electrochemical hydride generation in a miniaturized electrolytic flow cell coupled to microwave-induced plasma atomic emission spectrometry for the determination of selenium[J].Fresenius' Journal of Analytical Chemistry, 2001, 371:740-745. doi: 10.1007/s002160101040
CrossRef Google Scholar
|
| [51] |
Özmen B, Matysik F M, Bings N H, Broekaert J A C.Optimization and evaluation of different chemical and electrochemical hydride generation systems for the determination of arsenic by microwave plasma torch optical emission spectrometry[J].Spectrochimica Acta Part B: Atomic Spectroscopy, 2004, 59:941-950. doi: 10.1016/j.sab.2004.04.006
CrossRef Google Scholar
|
| [52] |
Boleaa E, Labordaa F, Castilloa J R, Sturgeon R E.Electrochemical hydride generation for the simultaneous determination of hydride forming elements by inductively coupled plasma-atomic emission spectrometry[J].Spectrochimica Acta Part B: Atomic Spectroscopy, 2004, 59(4):505-513. doi: 10.1016/j.sab.2004.01.005
CrossRef Google Scholar
|
| [53] |
Pohla P, Zapata I J, Bings N H.Optimization and comparison of chemical and electrochemical hydride generation for optical emission spectrometric determination of arsenic and antimony using a novel miniaturized microwave induced argon plasma exiting the microstrip wafer[J].Analytica Chimica Acta, 2008, 606(1,7):9-18.
Google Scholar
|
| [54] |
Červeny V, Horváth M, Broekaert J A C.Determination of mercury in water samples by electrochemical cold vapor generation coupled to microstrip microwave induced helium plasma optical emission spectrometry[J].Microchemical Journal, 2013,107:10-16. doi: 10.1016/j.microc.2012.05.023
CrossRef Google Scholar
|
| [55] |
Arbab-Zavara M H, Chamsaza M, Youssefib A,Aliakbari M.Mechanistic aspects of electrochemical hydride generation for cadmium[J].Analytica Chimica Acta, 2006,576(2):215-220. doi: 10.1016/j.aca.2006.06.015
CrossRef Google Scholar
|
| [56] |
Martin A A, Nicolas H B, Pawel P, Jos A C B.Investigation of electrochemical hydride generation coupled to microwave plasma torch optical emission spectrometry for the determination of arsenic: Analytical figures of merit, interference studies and applications to environmentally relevant samples[J].International Journal of Environmental Analytical Chemistry, 2008, 88(9):625-636. doi: 10.1080/03067310801983765
CrossRef Google Scholar
|
| [57] |
Machado L F R, Jacintho A O, Menegario A A, Zagatto E A G, Gine M F.Electrochemical and chemical processes for hydride generation in flow injection ICP-MS: Determination of arsenic in natural waters[J].Journal of Analytical Atomic Spectrometry, 1998, 13(12): 1343-1346. doi: 10.1039/A806383D
CrossRef Google Scholar
|
| [58] |
Bings N H, Stefánka Z, Mallada S R.Flow injection electrochemical hydride generation inductively coupled plasma time-of-flight mass spectrometry for the simultaneous determination of hydride forming elements and its application to the analysis of fresh water samples[J].Analytica Chimica Acta, 2003, 479(2):203-214. doi: 10.1016/S0003-2670(02)01526-X
CrossRef Google Scholar
|





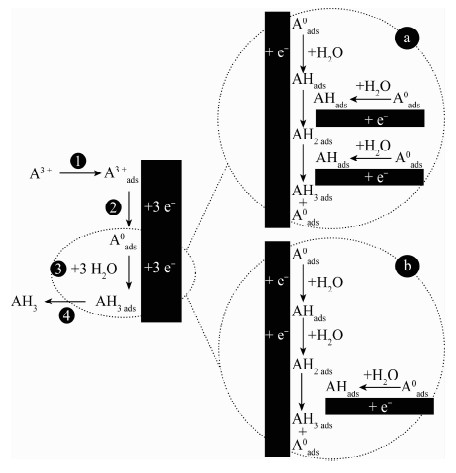

 DownLoad:
DownLoad: