| Citation: | Liang Wen, Zhou Nian-qing, Dai Chao-meng, Duan Yan-ping, Zhou Lang, Tu Yao-jen. 2021. Study of diclofenac removal by the application of combined zero-valent iron and calcium peroxide nanoparticles in groundwater. Journal of Groundwater Science and Engineering, 9(3): 171-180. doi: 10.19637/j.cnki.2305-7068.2021.03.001 |
Study of diclofenac removal by the application of combined zero-valent iron and calcium peroxide nanoparticles in groundwater
-
Abstract
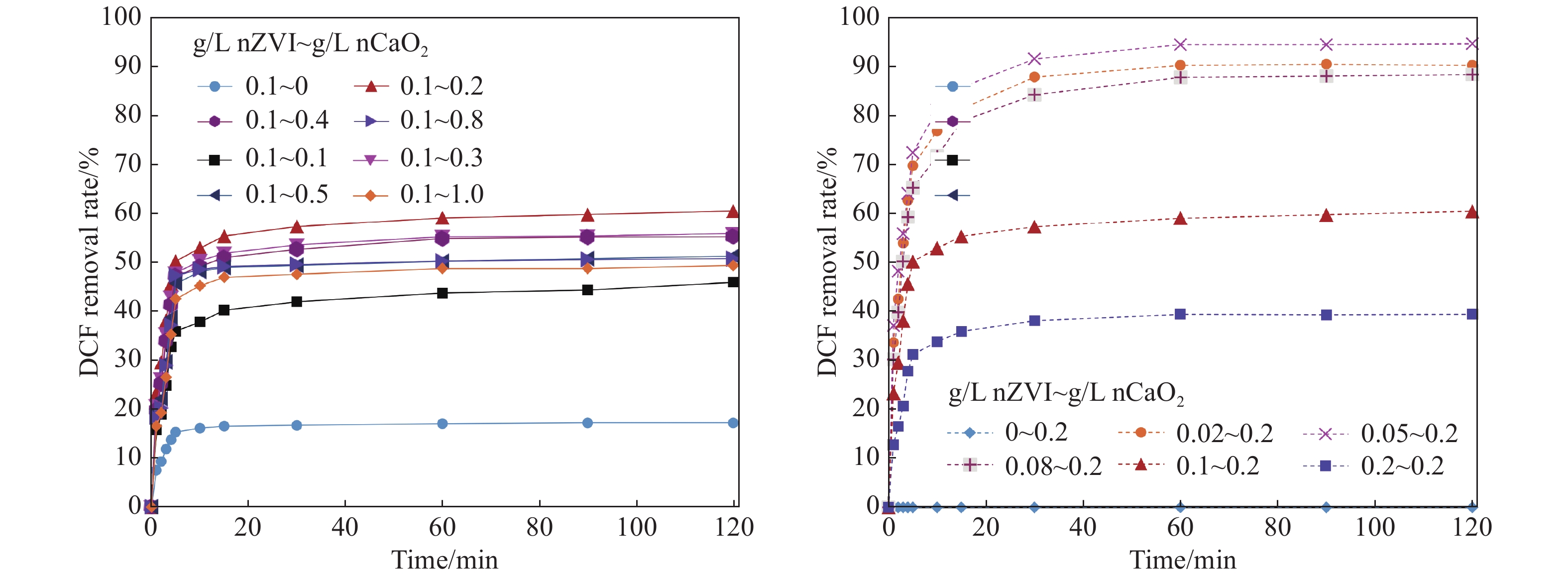
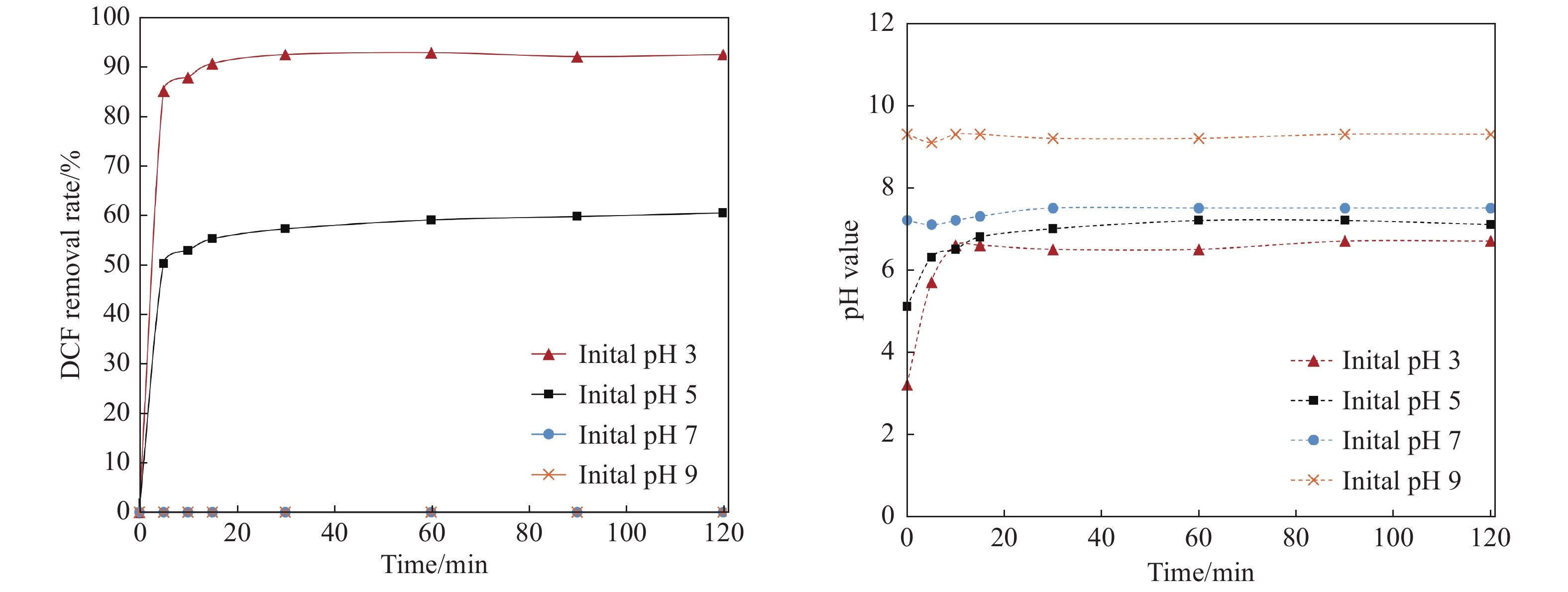
Diclofenac (DCF) is one of the most frequently detected pharmaceuticals in groundwater, posing a great threat to the environment and human health due to its toxicity. To mitigate the DCF contamination, experiments on DCF degradation by the combined process of zero-valent iron nanoparticles (nZVI) and nano calcium peroxide (nCaO2) were performed. A batch experiment was conducted to examine the influence of the adding dosages of both nZVI and nCaO2 nanoparticles and pH value on the DCF removal. In the meantime, the continuous-flow experiment was done to explore the sustainability of the DCF degradation by jointly adding nZVI/nCaO2 nanoparticles in the reaction system. The results show that the nZVI/nCaO2 can effectively remove the DCF in the batch test with only 0.05 g/L nZVI and 0.2 g/L nCaO2 added, resulting in a removal rate of greater than 90% in a 2-hour reaction with an initial pH of 5. The degradation rate of DCF was positively correlated with the dosage of nCaO2, and negatively correlated with both nZVI dosage and the initial pH value. The order of significance of the three factors is identified as pH value > nZVI dosage > nCaO2 dosage. In the continuous-flow reaction system, the DCF removal rates remained above 75% within 150 minutes at the pH of 5, with the applied dosages of 0.5 g/L for nZVI and 1.0 g/L for nCaO2. These results provide a theoretical basis for the nZVI/nCaO2 application to remove DCF in groundwater.
-

-
References
[1] Abraham N, Daniel C. 2008. Calcium peroxide (CaO2) for use in modified Fenton chemistry. Journal of Hazardous Materials, 152(3): 1164-1170. doi: 10.1016/j.jhazmat.2007.07.096 [2] Bin W, Shu-bo D, Jun H, et al. 2013. Environmental risk assessment and control of emerging contaminants in China. Environmental Chemistry(7): 1129-1136. doi: 10.7524/j.issn.0254-6108.2013.07.003 [3] Einsiedl F, Radke M, Maloszewski P. 2010. Occurrence and transport of pharmaceuticals in a karst groundwater system affected by domestic wastewater treatment plants. Journal of Contaminant Hydrology, 117(1): 26-36. doi: 10.1016/j.jconhyd.2010.05.008 [4] Forrez I, Carballa M, Verbeken K, et al. 2010. Diclofenac oxidation by biogenic manganese oxides. Environmental Science and Technology, 44(9): 3449-3454. doi: 10.1021/es9027327 [5] Gomes DS, Gando-Ferreira LM, Quinta-Ferreira RM, et al. 2018. Removal of sulfamethoxazole and diclofenac from water: Strategies involving O3 and H2O2. Environmental Technology, 39(13): 1658-1669. doi: 10.1080/09593330.2017.1335351 [6] Joo SH, FEITZ AJ, WAITE TD. 2004. Oxidative degradation of the carbothioate herbicide, molinate, using nanosoale zero-valent iron. Environmental Science and Technology, 38(7): 2242-2247. doi: 10.1021/es035157g [7] Jurado A, Walther M, Díaz-Cruz MS. 2019. Occurrence, fate and environmental risk assessment of the organic microcontaminants included in the Watch Lists set by EU Decisions 2015/495 and 2018/840 in the groundwater of Spain. Science of the Total Environment, 663: 285-296. doi: 10.1016/j.scitotenv.2019.01.270 [8] Li R, Jin X, Megharaj M, et al. 2015. Heterogeneous Fenton oxidation of 2,4-dichlorophenol using iron-based nanoparticles and persulfate system. Chemical Engineering Journal, 264: 587-594. doi: 10.1016/j.cej.2014.11.128 [9] Liang W, Dai CM, Zhou XF, et al. 2014. Application of zero-valent iron nanoparticles for the removal of aqueous zinc ions under various experimental conditions. Plos One, 9(1): e85686. doi: 10.1371/journal.pone.0085686 [10] Liang W, Zhou NQ, Dai C M, et al. 2020. Zero-valent iron nanoparticles and its combined process for diclofenac degradation under various experimental conditions. Polish Journal of Environmental Studies, 30(2): 1279-1288. doi: 10.15244/pjoes/123921 [11] Mikhailov I, Komarov S, Levina V, et al. 2017. Nanosized zero-valent iron as Fenton-like reagent for ultrasonic-assisted leaching of zinc from blast furnace sludge. Journal of Hazardous Materials, 321: 557-565. doi: 10.1016/j.jhazmat.2016.09.046 [12] Muhammad U, Hamidi Abdul A, Mohd Suffian Y. 2010. Trends in the use of Fenton, electro-Fenton and photo-Fenton for the treatment of landfill leachate. Waste Management, 30(11): 2113-2121. doi: 10.1016/j.wasman.2010.07.003 [13] Mutiyar PK, Gupta SK, Mittal AK. 2018. Fate of pharmaceutical active compounds (PhACs) from River Yamuna, India: An ecotoxicological risk assessment approach. Ecotoxicology and Environmental Safety, 150(15): 297-304. doi: 10.1016/j.ecoenv.2017.12.041 [14] Qian Y, Zhou X, Zhang Y, et al. 2013. Performance and properties of nanoscale calcium peroxide for toluene removal. Chemosphere, 91(5): 717-723. doi: 10.1016/j.chemosphere.2013.01.049 [15] Schwaiger J, Ferling H, Mallow U, et al. 2004. Toxic effects of the non-steroidal anti-inflammatory drug diclofenac: Part I: Histopathological alterations and bioaccumulation in rainbow trout. Aquatic Toxicology, 68(2): 141-150. doi: 10.1016/j.aquatox.2004.03.014 [16] Shen J, Ou C, Zhou Z, et al. 2013. Pretreatment of 2,4-dinitroanisole (DNAN) producing wastewater using a combined zero-valent iron (ZVI) reduction and Fenton oxidation process. Journal of Hazardous Materials, 260: 993-1000. doi: 10.1016/j.jhazmat.2013.07.003 [17] Shirazi E, Torabian A, Nabi Bidhendi G. 2013. Carbamazepine removal from groundwater: Effectiveness of the TiO2/UV, nanoparticulate zero-valent iron, and Fenton (nZVI/H2O2) processes. Clean - Soil Air Water, 41(11): 1062-1072. doi: 10.1002/clen.201200222 [18] Singh R, Misra V, Mudiam MKR, et al. 2012. Degradation of γ-HCH spiked soil using stabilized Pd/Fe0 bimetallic nanoparticles: Pathways, kinetics and effect of reaction condition. Journal of Hazardous Materials, 237-238: 355-364. doi: 10.1016/j.jhazmat.2012.08.064 [19] Stefaniuk M, Oleszczuk P, Ok YS. 2016. Review on nano zerovalent iron (nZVI): From synthesis to environmental applications. Chemical Engineering Journal, 287: 618-632. doi: 10.1016/j.cej.2015.11.046 [20] Sun YP, Li XQ, Cao J, et al. 2006. Characterization of zero-valent iron nanoparticles. Advances in Colloid and Interface Science, 120(1-3): 47-56. doi: 10.1016/j.cis.2006.03.001 [21] Vieno N, Sillanpää M. 2014. Fate of diclofenac in municipal wastewater treatment plant – A review. Environment International, 69: 28-39. doi: 10.1016/j.envint.2014.03.021 [22] Vilardi G, Sebastiani D, Miliziano S, et al. 2018. Heterogeneous nZVI-induced Fenton oxidation process to enhance biodegradability of excavation by-products. Chemical Engineering Journal, 335: 309-320. doi: 10.1016/j.cej.2017.10.152 [23] Vymazal J, Dvořáková Březinová T, Koželuh M, et al. 2017. Occurrence and removal of pharmaceuticals in four full-scale constructed wetlands in the Czech Republic–the first year of monitoring. Ecological Engineering, 98: 354-364. doi: 10.1016/j.ecoleng.2016.08.010 [24] Wang HF, Zhao YS, Li TY, et al. 2016. Properties of calcium peroxide for release of hydrogen peroxide and oxygen: A kinetics study. Chemical Engineering Journal, 303: 450-457. doi: 10.1016/j.cej.2016.05.123 [25] Zhang W, Gao H, He J, et al. 2017. Removal of norfloxacin using coupled synthesized nanoscale zero-valent iron (nZVI) with H2O2 system: Optimization of operating conditions and degradation pathway. Separation and Purification Technology, 172: 158-167. doi: 10.1016/j.seppur.2016.08.008 -
Access History

-
Figure 1.
TEM characterization of fresh nZVI
-
Figure 2.
XRD spectrum of fresh nZVI
-
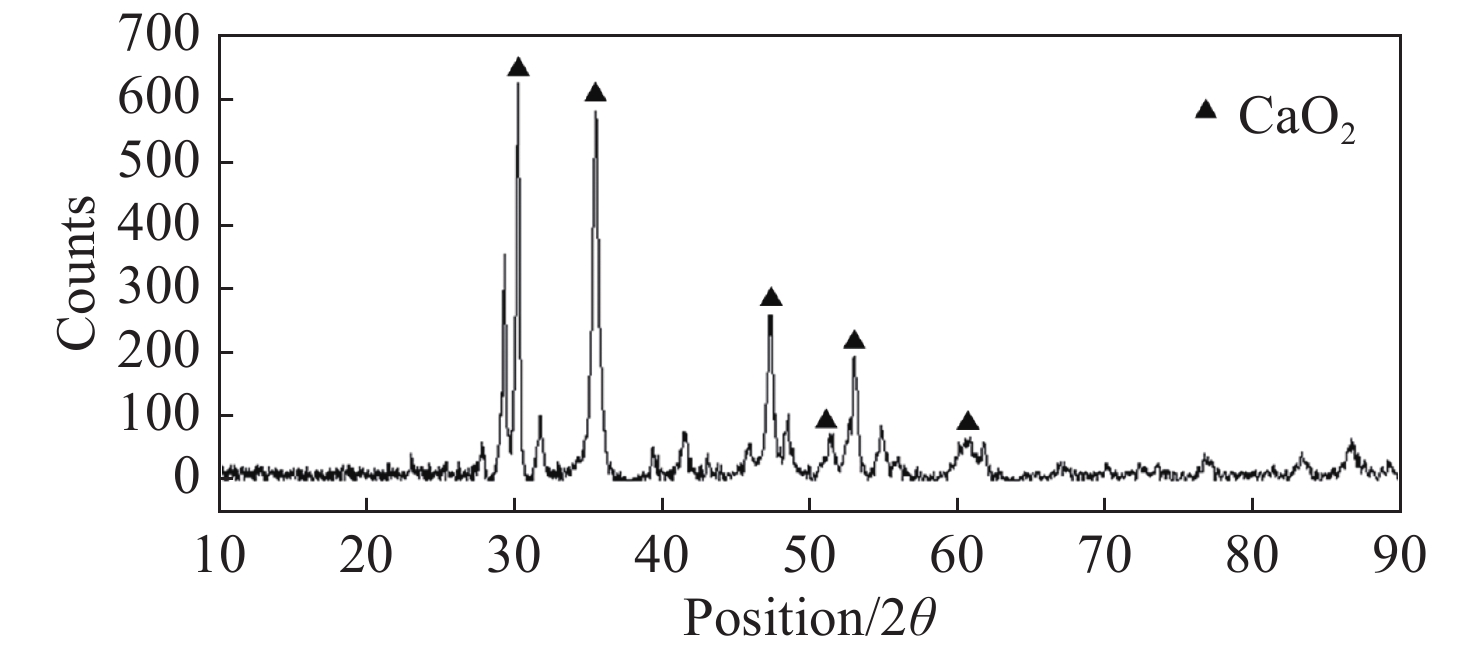
Figure 3.
XRD spectrum of fresh nCaO2
-
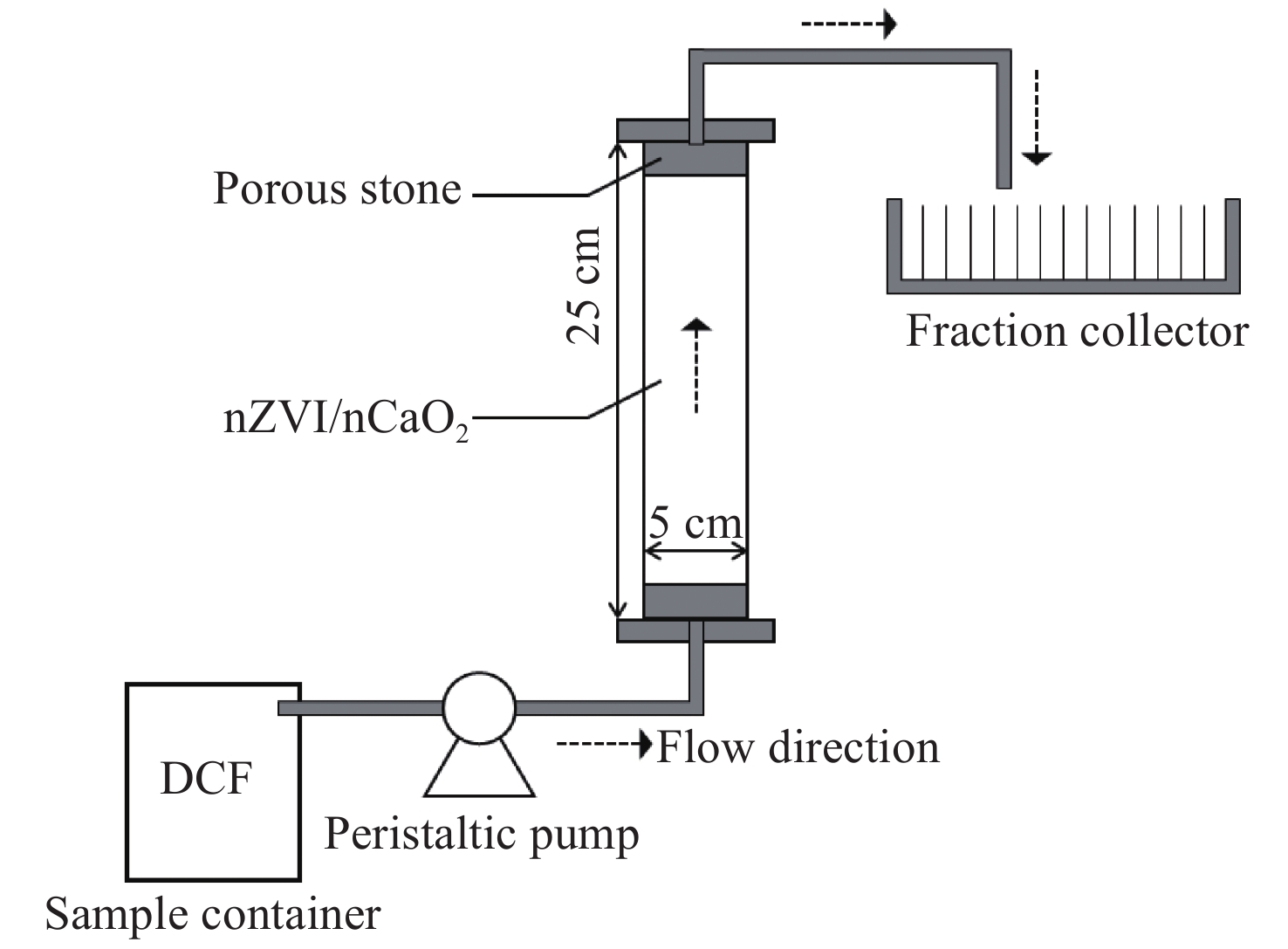
Figure 4.
The continuous-flow reactor
-
Figure 5.
Effect of nZVI/nCaO2 dosages on DCF removal
-
Figure 6.
Effect of initial pH on DCF removal by nZVI/nCaO2
-
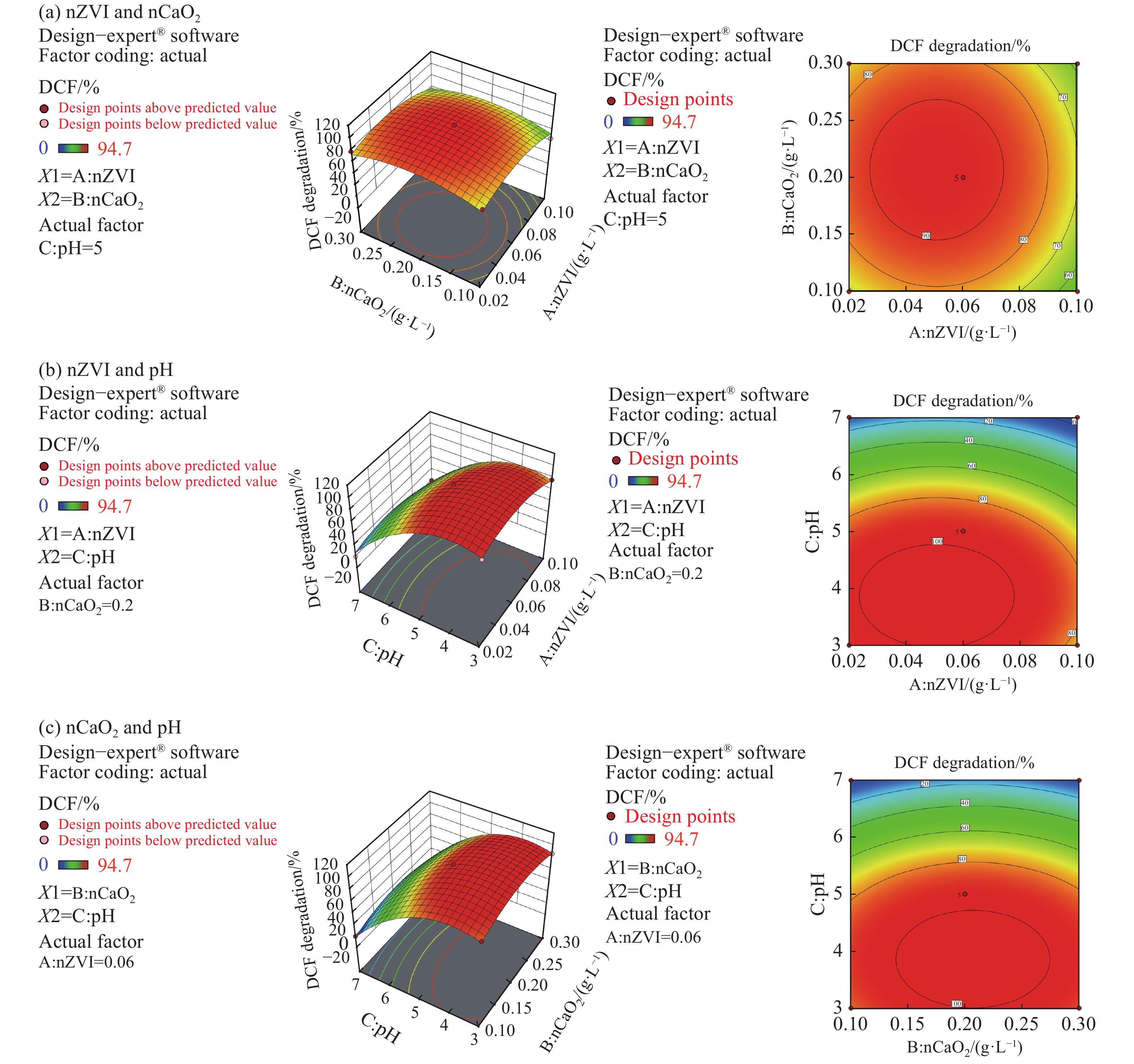
Figure 7.
3-D surfaces and contour plots for the effect of factors on DCF degradation
-
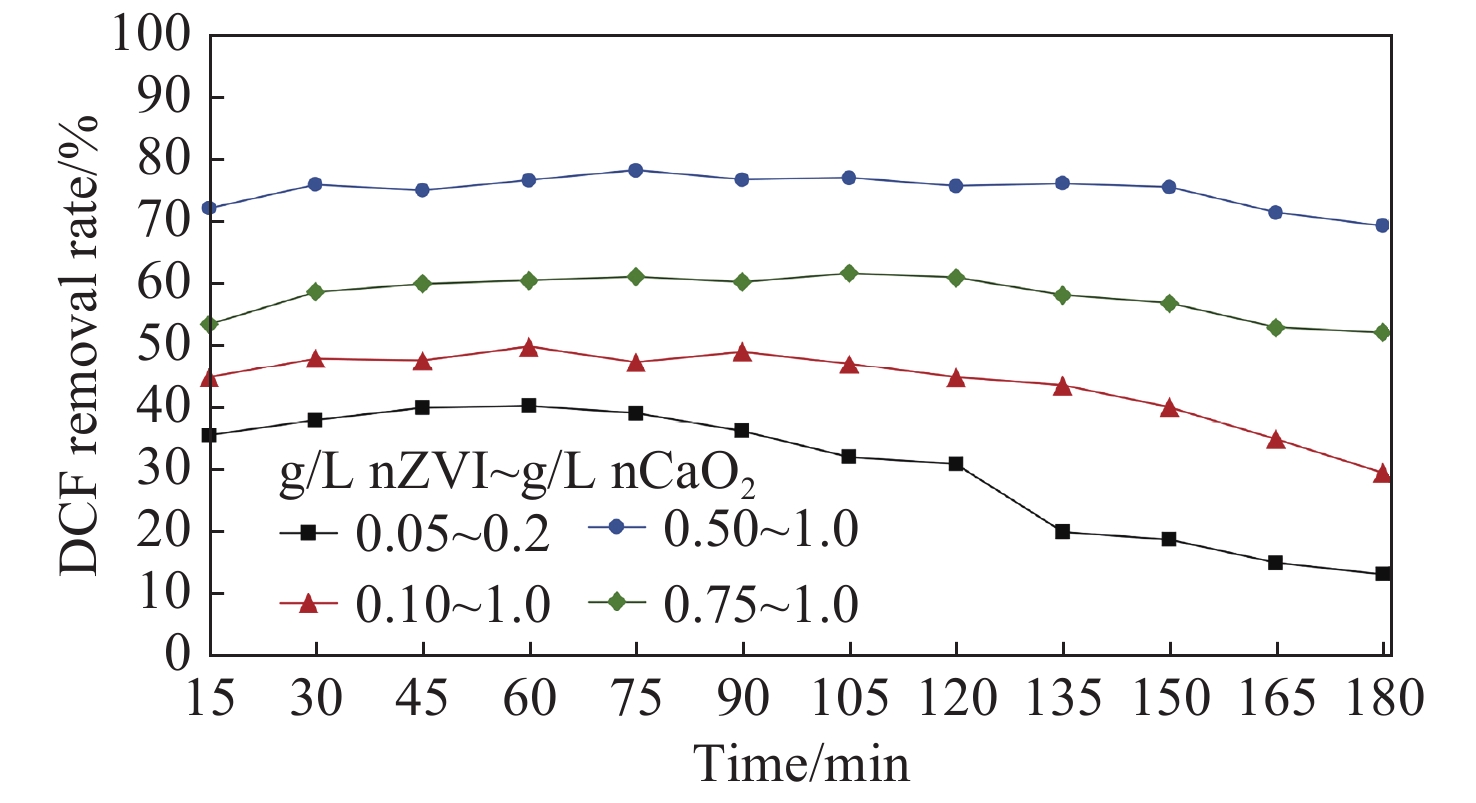
Figure 8.
DCF degradation by nZVI/nCaO2 in continuous-flow experiments





 DownLoad:
DownLoad: