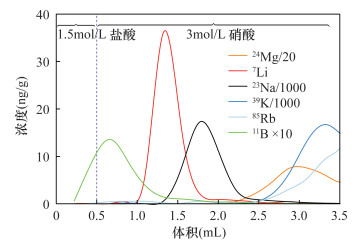
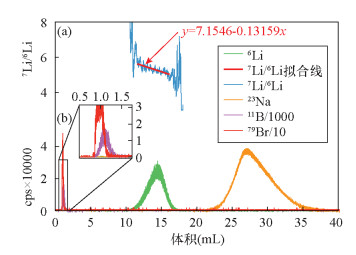
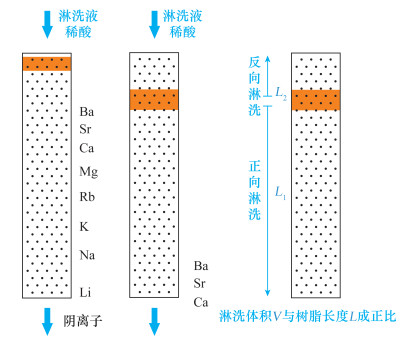
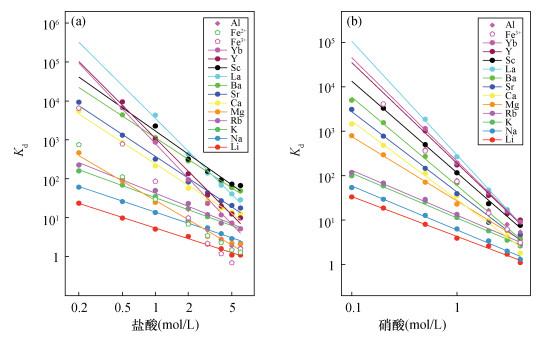
| [1] |
Ireland T J, Tissot F L H, Yokochi R, et al.Teflon-HPLC:A novel chromatographic system for application to isotope geochemistry and other industries[J].Chemical Geology, 2013, 357:203-214.
Google Scholar
|
| [2] |
Romaniello S J, Field M P, Smith H B, et al.Fully automated chromatographic purification of Sr and Ca for isotopic analysis[J].Journal of Analytical Atomic Spectrometry, 2015, 30:1906-1912.
Google Scholar
|
| [3] |
Retzmann A, Zimmermann T, Pröfrock D, et al.A fully automated simultaneous single-stage separation of Sr, Pb, and Nd using DGA resin for the isotopic analysis of marine sediments[J].Analytical and Bioanalytical Chemistry, 2017, 409:1-18.
Google Scholar
|
| [4] |
Wuttig K, Townsend A T, van der Merwe P, et al.Critical evaluation of a sea FAST system for the analysis of trace metals in marine samples[J].Talanta, 2019, 197:653-668.
Google Scholar
|
| [5] |
Zhu Z Y, Yang T, Zhu X K.Achieving rapid analysis of Li isotopes in high- matrix and low-Li samples with MC-ICP-MS:New developments in sample preparation and mass bias behavior of Li in ICPMS[J].Journal of Analytical Atomic Spectrometry, 2019, 34:1503-1513.
Google Scholar
|
| [6] |
Zhu Z Y, Jiang S Y, Yang T, et al.Improvements in Cu-Zn isotope analysis with MC-ICP-MS:A revisit of chemical purification, mass spectrometry measurement and mechanism of Cu/Zn mass bias decoupling effect[J].International Journal of Mass Spectrometry, 2015, 393:34-40.
Google Scholar
|
| [7] |
刘纯瑶, 苟龙飞, 邓丽, 等.离子交换过程中锂同位素分馏对锂同位素测试准确度的影响[J].岩矿测试, 2019, 38(1):35-44.
Google Scholar
Liu C Y, Gou L F, Deng L, et al.Effects of Li isotopic fractionation during ion exchange on the measurement accuracy of Li isotopes[J].Rock and Mineral Analysis, 2019, 38(1):35-44.
Google Scholar
|
| [8] |
濮魏, 高剑锋, 凌洪飞, 等.利用DCTA和HIBA快速有效分离Rb-Sr、Sm-Nd的方法[J].南京大学学报(自然科学版), 2005, 41(2):445-450.
Google Scholar
Pu W, Gao J F, Ling H F, et al.Separation method of Rb-Sr, Sm-Nd using DCTA and HIBA[J].Journal of Nanjing University (Natural Sciences), 2005, 41(2):445-450.
Google Scholar
|
| [9] |
尹鹏, 何倩, 何会军, 等.离子交换树脂法分离沉积物中锶和钕的影响因素研究[J].岩矿测试, 2018, 37(4):379-387.
Google Scholar
Yin P, He Q, He H J, et al.Study on the factors influencing the separation of Sr and Nd in sediments by ion exchange resin[J].Rock and Mineral Analysis, 2018, 37(4):379-387.
Google Scholar
|
| [10] |
唐索寒, 李津, 梁细荣, 等.钕同位素比值143Nd/144Nd标准溶液研制[J].岩矿测试, 2017, 36(2):163-170.
Google Scholar
Tang S H, Li J, Liang X R, et al.Reference material preparation of 143Nd/144Nd isotope ratio[J].Rock and Mineral Analysis, 2017, 36(2):163-170.
Google Scholar
|
| [11] |
张乐, 任钟元, 丁相礼, 等.微钻取样-TIMS/MC-ICPMS和LA-MC-ICPMS分析矿物岩石87Sr/86Sr比值的技术比较[J].岩矿测试, 2014, 33(5):615-624.
Google Scholar
Zhang L, Ren Z Y, Ding X L, et al.A comparison of microdrilling-TIMS/MC-ICPMS and LA-MC-ICPMS for micro-sample Sr isotope measurement[J].Rock and Mineral Analysis, 2014, 33(5):615-624.
Google Scholar
|
| [12] |
Glennon K J, Osborn J M, Burns J D, et al.Measuring key Sm isotope ratios in irradiated UO2 for use in plutonium discrimination nuclear forensics[J].Journal of Radioanalytical and Nuclear Chemistry, 2019, 320(2):405-414.
Google Scholar
|
| [13] |
Garcia-Valls R, Hrdlicka A, Perutka J, et al.Separation of rare earth elements by high performance liquid chromatography using a covalent modified silica gel column[J]. Analytica Chemica Acta, 2001, 439:247-253.
Google Scholar
|
| [14] |
李立武, 刘艳, 王先彬, 等.高真空与脉冲放电气相色谱联用装置研发及其在岩石脱气化学分析中的应用[J].岩矿测试, 2017, 36(3):222-230.
Google Scholar
Li L W, Liu Y, Wang X B, et al.Development of a combined device with high vacuum and pulsed discharge gas chromatography and its application in chemical analysis of gases from rock samples[J].Rock and Mineral Analysis, 2017, 36(3):222-230.
Google Scholar
|
| [15] |
Schwantes J M, Rundberg R S, Taylor W A, et al.Rapid, high-purity, lanthanide separations using HPLC[J].Journal of Alloys and Compounds, 2006, 418(1-2):189-194.
Google Scholar
|
| [16] |
Datta A, Sivaraman N, Srinivasan T G, et al.Single- stage dual-column HPLC technique for separation and determination of lanthanides in uranium matrix:Application to burnup measurement on nuclear reactor fuel[J].Nuclear Technology, 2013, 182(1):84-97.
Google Scholar
|
| [17] |
Liu X M, Li W S.Optimization of lithium isotope analysis in geological materials by quadrupole ICP-MS[J].Journal of Analytical Atomic Spectrometry, 2019, 34(8):1708-1717.
Google Scholar
|
| [18] |
Brugger J, McPhail D C, Black J, et al.Complexation of metal ions in brines:Application of electronic spectroscopy in the study of the Cu(Ⅱ)-LiCl-H2O system between 25℃ and 90℃[J].Geochimica et Cosmochimica Acta, 2001, 65(16):2691-2708.
Google Scholar
|
| [19] |
Bjerrum J, Lukes I.The iron(Ⅲ)-chloride system.A study of the stability constants and of the distribution of the tetrachloro species between organic solvents and aqueous chloride solutions[J].Acta Chemica Scandinavica, 1986, A40:31-40.
Google Scholar
|
| [20] |
Lee S G, Tanaka T.Determination of Eu isotopic ratio by multi-collector inductively coupled plasma mass spectrometry using a Sm internal standard[J].Spectrochimica Acta Part B:Atomic Spectroscopy, 2019, 156:42-50.
Google Scholar
|
| [21] |
Lee S G, Asahara Y, Tanaka T, et al.La-Ce and Sm-Nd isotopic systematics of Early Proterozoic leucogranite with tetrad REE pattern[J].Chemical Geology, 2010, 276(3-4):360-373.
Google Scholar
|
| [22] |
Panigrahi M, Grabda M, Kozak D, et al.Liquid-liquid extraction of neodymium ions from aqueous solutions of NdCl3 by phosphonium-based ionic liquids[J].Separation and Purification Technology, 2016, 171:263-269.
Google Scholar
|
| [23] |
Tanaka T, Lee S G, Kim T, et al.Precise determination of 14 REEs in GSJ/AIST geochemical reference materials JCp-1(coral) and JCt-1(giant clam) using isotope dilution ICP-quadrupole mass spectrometry[J].Geochemical Journal, 2018, 52(1):75-79.
Google Scholar
|
| [24] |
Colim A N, do Nascimento P C, Wiethan B A, et al.Reversed-phase high-performance liquid chromatography for the determination of 15 rare earth elements in surface water sample collected in a mining area from Lavras do Sul/RS, Brazil[J].Chromatographia, 2019, 82(5):843-856.
Google Scholar
|
| [25] |
Amr M A, Dawood N D A, Helal A I, et al.Rare earth elements and 143Nd/144Nd isotope ratio measurements using tandem ICP-CRC-MS/MS:Characterization of date palm (Phoenix dactylifera L.)[J].Journal of Analytical Atomic Spectrometry, 2017, 32(8):1554-1565.
Google Scholar
|
| [26] |
Lei H L, Yang T, Jiang S Y, et al.A simple two-stage column chromatographic separation scheme for strontium, lead, neodymium and hafnium isotope analyses in geological samples by thermal ionization mass spectrometry or multi-collector inductively coupled plasma mass spectrometry[J].Journal of Separation Science, 2019, 42(20):3261-3275.
Google Scholar
|
| [27] |
Golloch A.Handbook of rare earth elements[M].De Gruyter Press, 2017.
Google Scholar
|
| [28] |
Small H, Stevens T S, Bauman W C.Novel ion exchange chromatographic method using conductimetric detection[J].Analytical Chemistry, 1975, 47:1801-1809.
Google Scholar
|
| [29] |
Horwitz E P, Bloomquist C A.Chemical separations for super-heavy element searches in irradiated uranium targets[J].Journal of Inorganic and Nuclear Chemistry, 1975, 37:425-434.
Google Scholar
|
| [30] |
Pin C, Zalduegui J.Sequential separation of light rare-earth elements, thorium and uranium by miniaturized extraction chromatography:Application to isotopic analyses of silicate rocks[J].Analytica Chimica Acta, 1997, 339:79-89.
Google Scholar
|
| [31] |
Strelow F W E.Distribution coefficients and ion exchange behavior of 46 elements with a macroreticular cation exchange resin in hydrochloric acid[J].Analytical Chemistry, 1984, 56:1053-1056.
Google Scholar
|
| [32] |
Strelow F W E.An ion exchange selectivity scale of cations based on equilibrium distribution coefficients[J].Analytical Chemistry, 1960, 32:1185-1188.
Google Scholar
|






 DownLoad:
DownLoad: