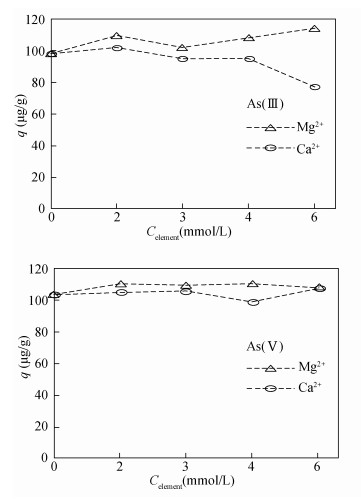
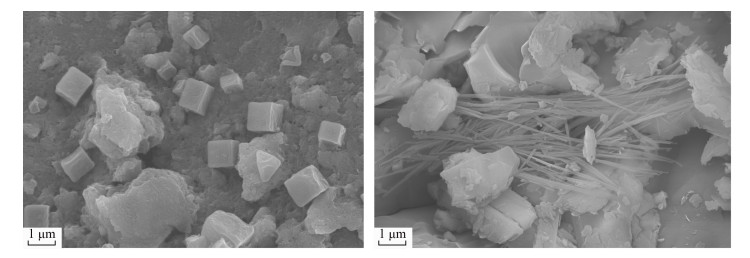
| [1] |
Mahanta R, Chowdhury J, Nath H K.Health costs of arsenic contamination of drinking water in Assam, India[J].Economic Analysis & Policy, 2016, 49:30-42.
Google Scholar
|
| [2] |
Ouédraogo I W K, Pehlivan E, Tran H T, et al.Removal of arsenic(Ⅴ) from aqueous medium using manganese oxide coated lignocellulose/silica adsorbents[J].Toxicological & Environmental Chemistry, 2015:1-21.
Google Scholar
|
| [3] |
Taleb K, Markovski J, Milosavljević M, et al.Efficient arsenic removal by cross-linked macroporous polymer impregnated with hydrous iron oxide:Material performance[J].Chemical Engineering Journal, 2015, 279:66-78. doi: 10.1016/j.cej.2015.04.147
CrossRef Google Scholar
|
| [4] |
Michael H A.An arsenic forecast for China[J].Science, 2013, 341(6148):852-853. doi: 10.1126/science.1242212
CrossRef Google Scholar
|
| [5] |
Rodríguez-Lado L, Sun G, Berg M, et al.Groundwater arsenic contamination throughout China[J].Science, 2013, 341(6148):866-868. doi: 10.1126/science.1237484
CrossRef Google Scholar
|
| [6] |
Baig S A, Sheng T, Hu Y, et al.Arsenic removal from natural water using low cost granulated adsorbents:A review[J].CLEAN-Soil, Air, Water, 2015, 43(1):13-26. doi: 10.1002/clen.201200466
CrossRef Google Scholar
|
| [7] |
Bora A J, Mohan R, Dutta R K.Simultaneous removal of arsenic, iron and manganese from groundwater by oxidation-coagulation-adsorption at optimized pH[J].Water Science & Technology Water Supply, 2017:ws2017092.
Google Scholar
|
| [8] |
Mohan D, Sharma R, Singh V K, et al.Fluoride removal from water using bio-char, a green waste, low-cost adsorbent:Equilibrium uptake and sorption dynamics modeling[J].Industrial & Engineering Chemistry Research, 2012, 51(2):900-914.
Google Scholar
|
| [9] |
Basu A, Saha D, Saha R, et al.A review on sources, toxicity and remediation technologies for removing arsenic from drinking water[J].Cheminform, 2014, 40(2):447-485.
Google Scholar
|
| [10] |
吴昆明, 郭华明, 魏朝俊.天然磁铁矿化学改性及其在水体除砷中的应用[J].岩矿测试, 2017, 36(1):32-39.
Google Scholar
Wu K M, Guo H M, Wei C J.Chemical modification of natural magnetite and its application in arsenic removal from water[J].Rock and Mineral Analysis, 2017, 36(1):32-39.
Google Scholar
|
| [11] |
Wang Y, Shen F, Qi X.A corn stalk-derived porous carbonaceous adsorbent for adsorption of ionic liquids from aqueous solution[J].RSC Advances, 2016, 39:32505-32513.
Google Scholar
|
| [12] |
Yamani J S, Lounsbury A W, Zimmerman J B.Towards a selective adsorbent for arsenate and selenite in the presence of phosphate:Assessment of adsorption efficiency, mechanism, and binary separation factors of the chitosan-copper complex[J].Water Research, 2016, 88:889. doi: 10.1016/j.watres.2015.11.017
CrossRef Google Scholar
|
| [13] |
Zhu N, Yan T, Qiao J, et al.Adsorption of arsenic, ph-osphorus and chromium by bismuth impregnated biochar:Adsorption mechanism and depleted adsorbent utilization[J].Chemosphere, 2016, 164:32-40. doi: 10.1016/j.chemosphere.2016.08.036
CrossRef Google Scholar
|
| [14] |
Lagergren S.About the theory of so-called adsorption of soluble substances[J].Kungliga Svenska Vetenskapsakademiens Handlingar Band, 1989, 24(4):1-39.
Google Scholar
|
| [15] |
Ho Y S, Mckay G.Pseudo-second order model for sorp-tion processes[J].Process Biochemistry, 1999, 34(5):451-465. doi: 10.1016/S0032-9592(98)00112-5
CrossRef Google Scholar
|
| [16] |
Lladó J, Lao-Luque C, Ruiz B, et al.Role of activated carbon properties in atrazine and paracetamol adsorption equilibrium and kinetics[J].Process Safety & Environmental Protection, 2015, 95:51-59.
Google Scholar
|
| [17] |
赵凯, 郭华明, 李媛, 等.天然菱铁矿改性及强化除砷研究[J].环境科学, 2012, 33(2):459-468.
Google Scholar
Zhao K, Guo H M, Li Y, et al.Modification of natural siderite and enhanced adsorption of arsenic[J].Environmental Science, 2012, 33(2):459-468.
Google Scholar
|
| [18] |
Qiao J, Jiang Z, Sun B, et al.Arsenate and arsenite re-moval by FeCl3:Effects of pH, As/Fe ratio, initial As concentration and co-existing solutes[J].Separation & Purification Technology, 2012, 92(1):106-114.
Google Scholar
|
| [19] |
Amstaetter K, Borch T, Laresecasanova P, et al.Redox transformation of arsenic by Fe(Ⅱ)-activated goethite (α-FeOOH)[J].Environmental Science & Technology, 2010, 44(1):102-108.
Google Scholar
|
| [20] |
Hcry M, Van Dongen B E, Gill F, et al.Arsenic release and attenuation in low organic carbon aquifer sediments from West Bengal[J].Geobiology, 2010, 8(2):155-168. doi: 10.1111/gbi.2010.8.issue-2
CrossRef Google Scholar
|





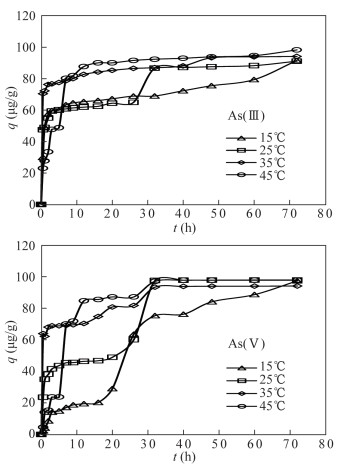

 DownLoad:
DownLoad: