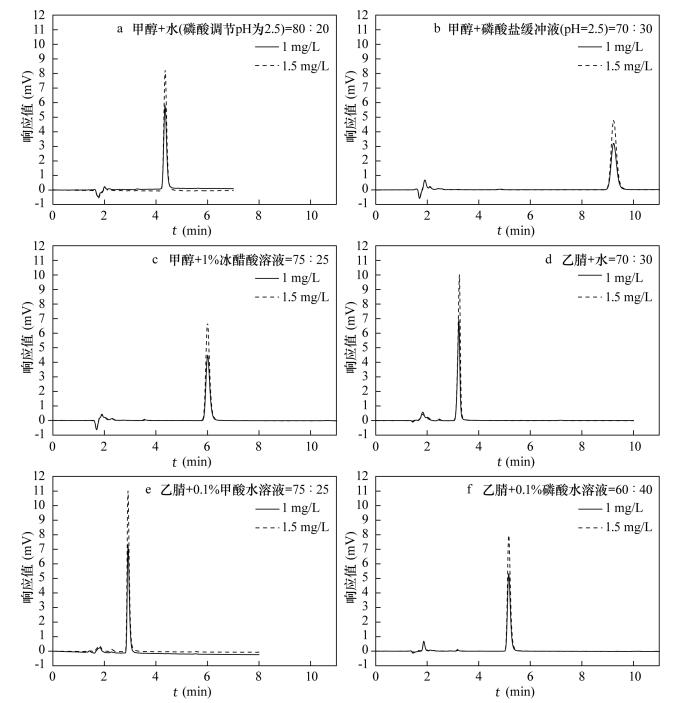
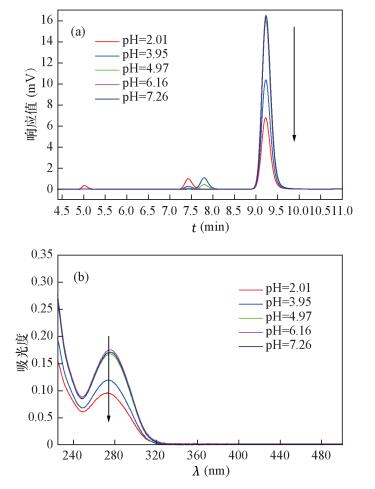
| [1] |
Vieno N, Sillanpää M.Fate of diclofenac in municipal wastewater treatment plant-A review[J].Environment International, 2014, 69:28-39. doi: 10.1016/j.envint.2014.03.021
CrossRef Google Scholar
|
| [2] |
Scheurell M, Franke S, Shah R M, et al.Occurrence of diclofenac and its metabolites in surface water and effluent samples from Karachi, Pakistan[J].Chemosphere, 2009, 77(6):870-876. doi: 10.1016/j.chemosphere.2009.07.066
CrossRef Google Scholar
|
| [3] |
Balakrishna K, Rath A, Praveenkumarreddy Y, et al.A review of the occurrence of pharmaceuticals and personal care products in Indian water bodies[J].Ecotoxicology and Environmental Safety, 2017, 137:113-120. doi: 10.1016/j.ecoenv.2016.11.014
CrossRef Google Scholar
|
| [4] |
López-Serna R, Jurado A, Vázquez-Suňé E, et al.Occurrence of 95 pharmaceuticals and transformation products in urban groundwaters underlying the metropolis of Barcelona, Spain[J].Environmental Pollution, 2013, 174:305-315. doi: 10.1016/j.envpol.2012.11.022
CrossRef Google Scholar
|
| [5] |
Carmona E, Andreu V, Picó Y.Occurrence of acidic pharmaceuticals and personal care products in Turia River Basin:From waste to drinking water[J].Science of the Total Environment, 2014, 484:53-63. doi: 10.1016/j.scitotenv.2014.02.085
CrossRef Google Scholar
|
| [6] |
温智皓, 段艳平, 孟祥周, 等.城市污水处理厂及其受纳水体中5种典型PPCPs的赋存特征和生态风险[J].环境科学, 2013, 34(3):927-932.
Google Scholar
Wen Z H, Duan Y P, Meng X Z, et al.Occurrence and risk assessment of five selected PPCPs in municipal wastewater treatment plant and the receiving water[J].Environmental Science, 2013, 34(3):927-932.
Google Scholar
|
| [7] |
Ma R, Wang B, Lu S, et al.Characterization of pharmaceutically active compounds in Dongting Lake, China:Occurrence, chiral profiling and environmental risk[J].Science of the Total Environment, 2016, 557:268-275.
Google Scholar
|
| [8] |
Ma R, Wang B, Yin L, et al.Characterization of pharmaceutically active compounds in Beijing, China:Occurrence pattern, spatiotemporal distribution and its environmental implication[J].Journal of Hazardous Materials, 2017, 323:147-155. doi: 10.1016/j.jhazmat.2016.05.030
CrossRef Google Scholar
|
| [9] |
Vulliet E, Cren-Olivé C.Screening of pharmaceuticals and hormones at the regional scale, in surface and groundwaters intended to human consumption[J].Environmental Pollution, 2011, 159(10):2929-2934. doi: 10.1016/j.envpol.2011.04.033
CrossRef Google Scholar
|
| [10] |
Feito R, Valcárcel Y, Catalá M.Biomarker assessment of toxicity with miniaturised bioassays:Diclofenac as a case study[J].Ecotoxicology, 2012, 21(1):289-296. doi: 10.1007/s10646-011-0790-2
CrossRef Google Scholar
|
| [11] |
Poirier-Larabie S, Segura P A, Gagnon C.Degradation of the pharmaceuticals diclofenac and sulfamethoxazole and their transformation products under controlled environmental conditions[J].Science of the Total Environment, 2016, 557:257-267.
Google Scholar
|
| [12] |
Schmitt-Jansen M, Bartels P, Adler N, et al.Phytotoxicity assessment of diclofenac and its phototransformation products[J].Analytical and Bioanalytical Chemistry, 2007, 387(4):1389-1396. doi: 10.1007/s00216-006-0825-3
CrossRef Google Scholar
|
| [13] |
Vedenyapina M D, Borisova D A, Simakova A P, et al.Adsorption of diclofenac sodium from aqueous solutions on expanded graphite[J].Solid Fuel Chemistry, 2013, 47(1):59-63. doi: 10.3103/S0361521912060134
CrossRef Google Scholar
|
| [14] |
Bajpai M, Rai N, Bajpai S K.Equilibrium adsorption studies on removal of diclofenac sodium from aqueous solution using sawdust-polyaniline (SD-PAn) composites[J].Journal of Applied Polymer Science, 2012, 125(2):1382-1390. doi: 10.1002/app.v125.2
CrossRef Google Scholar
|
| [15] |
朱小红, 李涛, 马鹏飞, 等.气相色谱-质谱检测方法快速筛查保健食品及中成药中8种非甾体抗炎[J].药物分析杂志, 2012, 32(10):1847-1852.
Google Scholar
Zhu X H, Li T, Ma P F, et al.GC-MS rapid screening of eight non-steroidal anti-inflammatorydrugs in health foods and traditional Chinese medicines[J].Chinese Journal of Pharmaceutical Analysis, 2012, 32(10):1847-1852.
Google Scholar
|
| [16] |
Kaynak M S, Buyutuncel E, Cagler H J, et al.Determination of regional intestinal permeability of diclofenac and metoprolol using a newly-developed and validated high performance liquid chromatographic method[J].Tropical Journal of Pharmaceutical Research, 2015, 14(1):163-170. doi: 10.4314/tjpr.v14i1.23
CrossRef Google Scholar
|
| [17] |
Sotelo J L, Ovejero G, Rodríguez A, et al.Competitive adsorption studies of caffeine and diclofenac aqueous solutions by activated carbon[J].Chemical Engineering Journal, 2014, 240:443-453. doi: 10.1016/j.cej.2013.11.094
CrossRef Google Scholar
|
| [18] |
Sotelo J L, Rodríguez A R, Mateos M M, et al.Adsorption of pharmaceutical compounds and an endocrine disruptor from aqueous solutions by carbon materials[J].Journal of Environmental Science and Health Part B, Pesticides, Food Contaminants, and Agricultural Wastes, 2012, 47(7):640-652. doi: 10.1080/03601234.2012.668462
CrossRef Google Scholar
|
| [19] |
张璟, 陈阳, 李沛, 等.基于微透析技术及HPLC-ESI-MS测定大鼠关节腔透析液中双氯芬酸钠浓度及其应用[J].中国临床药理学与治疗学, 2012, 17(11):1233-1239.
Google Scholar
Zhang J, Chen Y, Li P, et al.Determination of diclofenac sodium in dialysate of joint cavity in rats based on microdialysis and HPLC-ESI-MS and its application[J].Chinese Journal of Clinical Pharmacology and Therapeutics, 2012, 17(11):1233-1239.
Google Scholar
|
| [20] |
卢红选, 刘卫国.HPLC-MS/MS测定城市废水中的10种药物与个人护理用品[J].地球环境学报, 2016, 7(4):425-430.
Google Scholar
Lu H X, Liu W G.Determination of 10 pharmaceuticals and personal care products in waste water by HPLC-MS/MS[J].Journal of Earth Environment, 2016, 7(4):425-430.
Google Scholar
|
| [21] |
Krajišnik D, Daković A, Milojević M, et al.Properties of diclofenac sodium sorption onto natural zeolite modified with cetylpyridinium chloride[J].Colloids Surfaces B:Biointerfaces, 2011, 83(1):165-172. doi: 10.1016/j.colsurfb.2010.11.024
CrossRef Google Scholar
|
| [22] |
石建稳, 艾慧颖, 王旭, 等.TiO2/粉煤灰光催化降解双氯芬酸钠研究[J].环境科学学报, 2014, 34(2):370-376.
Google Scholar
Shi J W, Ai H Y, Wang X, et al.Photocatalytic degradation of diclofenac sodium over the photocatalyst of TiO2/CFA[J].Acta Scientiae Circumstantiae, 2014, 34(2):370-376.
Google Scholar
|
| [23] |
张楠, 刘国光, 刘海津, 等.双氯芬酸在水环境中光降解的初步研究[J].环境化学, 2013, 32(1):42-47. doi: 10.7524/j.issn.0254-6108.2013.01.007
CrossRef Google Scholar
Zhang N, Liu G G, Liu H J, et al.Photodegration mechanism of diclofenac in aqueous environment[J].Environmental Chemistry, 2013, 32(1):42-47. doi: 10.7524/j.issn.0254-6108.2013.01.007
CrossRef Google Scholar
|
| [24] |
于万禄, 熊振湖, 马华继.Photo-Fenton法降解水中新型污染物双氯芬酸及降解产物的毒性评价[J].环境科学学报, 2009, 29(10):2070-2075. doi: 10.3321/j.issn:0253-2468.2009.10.008
CrossRef Google Scholar
Yu W L, Xiong Z H, Ma H J.Degradation of the emergent pollutant diclofenac in water by photo-Fenton and toxicity evaluation of its degradation products[J].Acta Scientiae Circumstantiae, 2009, 29(10):2070-2075. doi: 10.3321/j.issn:0253-2468.2009.10.008
CrossRef Google Scholar
|
| [25] |
Huguet M, Deborde M, Papot S, et al.Oxidative decarboxylation of diclofenac by manganese oxide bed filter[J].Water Research, 2013, 47(14):5400-5408. doi: 10.1016/j.watres.2013.06.016
CrossRef Google Scholar
|
| [26] |
童新, 王军辉.纳米TiO2光催化氧化去除水中痕量双氯芬酸的研究[J].环境污染与防治, 2012, 34(8):53-57.
Google Scholar
Tong X, Wang J H.Photocatalytic oxidation of trace diclofenac in water by nanosized TiO2[J].Environmental Pollution & Control, 2012, 34(8):53-57.
Google Scholar
|
| [27] |
张楠. 双氯芬酸在水环境中光解行为的研究[D]. 新乡: 河南师范大学, 2012.http://cdmd.cnki.com.cn/Article/CDMD-10476-1012421584.htm
Google Scholar
Zhang N. Study on Photolytic Behavior of Diclofenac in Aqueous Environment[D]. Xinxiang: Henan Normal University, 2012.
Google Scholar
|
| [28] |
李璐, 刘菲, 陈鸿汉, 等.高效液相色谱法同时测定水体中的环丙沙星和氟甲喹[J].色谱, 2013, 31(6):567-571.
Google Scholar
Li L, Liu F, Chen H H, et al.Simultaneous determination of ciprofloxacin and flumequine in water samples by high performance liquid chromatography[J].Chinese Journal of Chromatography, 2013, 31(6):567-571.
Google Scholar
|
| [29] |
冯奇奇, 卜龙利, 高波, 等.ZnIn2S4可见光催化降解水中的双氯芬酸[J].环境工程学报, 2017, 11(2):739-747. doi: 10.12030/j.cjee.201509051
CrossRef Google Scholar
Feng Q Q, Bu L L, Gao B, et al.Photocatalytic degradation of aqueous diclofenac by ZnIn2S4 under visible light[J].Chinese Journal of Environmental Engineering, 2017, 11(2):739-747. doi: 10.12030/j.cjee.201509051
CrossRef Google Scholar
|
| [30] |
Chefetz B, Mualem T, Ben-Ari J.Sorption and mobility of pharmaceutical compounds in soil irrigated with reclaimed wastewater[J].Chemosphere, 2008, 73(8):1335-1343. doi: 10.1016/j.chemosphere.2008.06.070
CrossRef Google Scholar
|
| [31] |
Wang Y, Liu H, Liu G, et al.Oxidation of diclofenac by potassium ferrate(Ⅵ):Reaction kinetics and toxicity evaluation[J].Science of the Total Environment, 2015, 506:252-258.
Google Scholar
|
| [32] |
Wang X, Li J R, Fu M L, et al.Fabrication and evaluation of Au-Pd core-shell nanocomposites for dechlorination of diclofenac in water[J].Environmental Technology, 2015, 36(12):1510-1518. doi: 10.1080/09593330.2014.994044
CrossRef Google Scholar
|
| [33] |
Wang Y, Liu H, Liu G, et al.Oxidation of diclofenac by aqueous chlorine dioxide:Identification of major disinfection by products and toxicity evaluation[J].Science of the Total Environment, 2014, 473:437-445.
Google Scholar
|
| [34] |
Kovala-Demertzi D.Transition metal complexes of di-clofenac with potentially interesting anti-inflammatory activity[J].Journal of Inorganic Biochemistry, 2000, 79(1):153-157.
Google Scholar
|





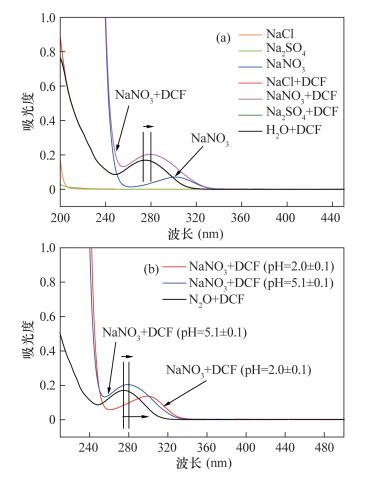

 DownLoad:
DownLoad: